The in situ monitoring of the transformation of moxidectin ethanol solvate to form I in an ethanol–water mixture
Abstract
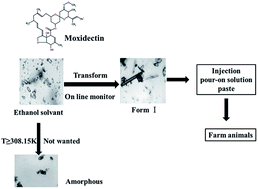
Moxidectin is a single-component and semisynthetic macrocyclic lactone antibiotic, which has been widely used in the prevention and treatment of parasites in farm animals. In this paper, the transformation of ethanol solvate to form I of moxidectin in an ethanol–water mixture was studied. Offline methods and online instruments were used to monitor and identify the transformation process, and the influences of water content and temperature were discussed. It is noted that the transformation kinetics are highly sensitive to both the solvent composition and temperature and the transformation rate is a function of the ethanol content in aqueous ethanol mixtures. The solvent-mediated polymorphic transformation mechanism from ethanol solvate to form I was suggested, and the process is controlled by the nucleation and growth rate of the stable form. Understanding these effects can aid optimization and improve process control in the crystallization of moxidectin.

 Please wait while we load your content...
Please wait while we load your content...