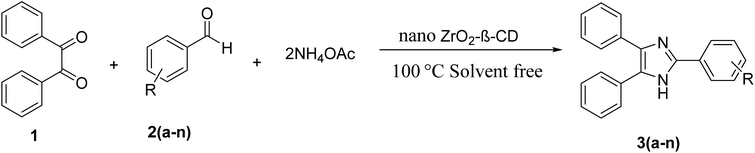
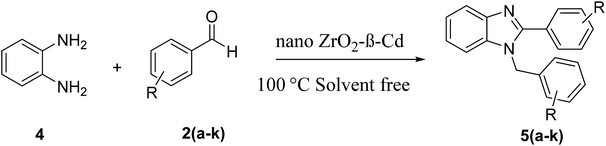
ZrO2-β-cyclodextrin catalyzed synthesis of 2,4,5-trisubstituted imidazoles and 1,2-disubstituted benzimidazoles under solvent free conditions and evaluation of their antibacterial study†
Yarabhally R. Girisha,
Kothanahally S. Sharath Kumara,
Kuntebommanahalli N. Thimmaiah*b,
Kanchugarakoppal S. Rangappa*a and
Sheena Shashikanth*a
aDepartment of Studies in Chemistry, Manasagangotri, University of Mysore, Mysore-570006, India. E-mail: shashis1956@gmail.com; Tel: +91-821-2419668
bDepartment of Chemistry, Northwest Mississippi Community College/University of Mississippi, DeSoto Center, Southaven, Mississippi-38671, USA
First published on 28th August 2015
Abstract
A series of 2,4,5-trisubstituted imidazoles and 1,2-disubstituted benzimidazoles catalyzed by ZrO2-supported-β-cyclodextrin (ZrO2-β-CD) under solvent free conditions have been synthesized and characterized by spectral methods. The nanoparticles (ZrO2-β-CD), prepared by a simple one-pot-coprecipitation method and were characterized by PXRD, SEM, and TEM techniques. The nano (ZrO2-β-CD) particles were found to be an effective heterogeneous reusable catalyst for the effective synthesis of imidazoles and benzimidazoles under solvent free conditions and all of the synthesized derivatives were evaluated for their antibacterial activity against six bacterial strains.
Introduction
Imidazole, an important class of five membered nitrogen containing heterocycles, play a vital role in the synthesis of biologically active molecules. These compounds are known to possess diverse biological applications as inhibitors of the transforming growth factor-β type I receptor (ALK5), mGAT3 selective GABA uptake inhibitors, anti-inflammatory agents, anti-cancer agents, fungicides, antithrombotic agents, anti-alzheimer's agents, and for the determination of propyl gallate in edible oil.1 These compounds also play an important role as a parent π-conjugated backbone for organic chromophores for the intramolecular charge-transfer process,2 for enhanced charge transfer for hetero-junction solar cell applications,3 and as a new building block for blue light emitting materials.4 Imidazole-based heterocycles have also been used for energetic materials.5 In addition phosphino imidazole and imidazolium salts have been used for the synthesis of Suzuki C–C coupling reactions.6Benzimidazole derivatives have drawn considerable attention due to their widespread biological applications such as antitumor,7 antimicrobial,8 anti-inflammatory,9 antihelminthic10 activities. In addition, benzimidazoles are important intermediates in various organic reactions.11–13 Therefore, the synthesis of benzimidazoles has gained much importance in recent years. Several synthetic efforts have been made for the synthesis of benzimidazoles using various catalysts.14–21
The conventional methods for the synthesis of 2,4,5-trisubstituted imidazole are mainly based on the cyclo condensation of a 1,2-diketone with an aldehyde using ammonium acetate as the nitrogen source.22 Various catalysts under different reaction conditions have been employed for the synthesis of imidazole based derivatives.23 Most of the reported methods suffer from one or the other serious drawbacks such as high reaction temperature, long duration, use of toxic and expensive chemicals as starting materials, use of moisture-sensitive catalysts and formation of byproducts. Recently nanoparticles have been extensively used in the field of medicinal chemistry,24,25 heterocyclic chemistry,26,27 as sensors for detection of hydrazine,28 optoelectronic materials,29 in enhancing the up-conversion luminescence,30 and as electron transfer mediators in a bio-electrochemical systems.31,32
Cyclodextrins (CDs) are natural oligosaccharides linked by α-1,4-glycosidic linkage, having hydrophobic cavities inside and hydrophilic outside. The substrates can be entrapped in the hydrophobic cavity of the cyclodextrin felicitating the catalysis of the chemical reactions for higher selectivity. β-Cyclodextrins have been extensively used in various biochemical applications such as for drug and gene delivery,33 to detect micromolar quantities of Pb2+ in aqueous solution,34 for removing diazepam from blood,35 for optical sensing and chiroselective sensing of different substrates using a fluorescence resonance energy transfer (FRET).36 In addition, β-cyclodextrin has also been used as catalyst for the synthesis of various heterocycles.37
In recent years ZrO2 nanoparticles have gained much attention in catalysis due to their specific amphoteric properties, excellent mechanical strength and stiffness, high thermal stability and dielectric properties.38–40 ZrO2 can exist in three polymorphic forms depending on the temperature range namely monoclinic (room temperature-1172 °C), tetragonal (1172–2347 °C) and cubic (above 2347 °C).41,42 ZrO2 have found wide spread applications in the field of science and technology.43–54
In continuation of our efforts in nanoparticles catalyzed synthesis of diverse nitrogen containing heterocycles,55 we have described ZrO2-β-CD catalyzed synthesis of imidazole and 1,2-disubstituted benzimidazoles from readily available benzyl, 1,2-phenylenediamine, aldehydes and ammonium acetate under solvent free conditions (Scheme 1) using ZrO2-β-CD as an environment friendly and reusable heterogeneous catalyst (Scheme 1). It is note-worthy to mention that, ZrO2-supported β-cyclodextrin nanoparticles have never been used in the field of synthetic organic chemistry for the synthesis of any heterocycles.
Results and discussion
The ZrO2 nanoparticles-supported β-cyclodextrin nanoparticles (ZrO2-β-CD) was prepared by a simple one-pot co-precipitation method by using ZrOCl2·8H2O, and NH4OH (Scheme 2).56 The morphology of the prepared ZrO2-β-CD nanoparticles were characterized by ATR-IR, PXRD, SEM, TEM and EDAX analysis, which confirmed the successful preparation of the ZrO2-supported β-cyclodextrin nanoparticles.Fig. 1 shows the ATR-IR spectrum of ZrO2-β-cyclodextrin nanoparticles. The bands observed at 844 and 510 cm−1, which is due to asymmetric and symmetric stretching modes of ZrO2. The broad band appears at 3337 cm−1 is for hydroxyl stretching vibration of β-cyclodextrin. The peak at 1348 corresponds to C–C stretching vibrations of β-cyclodextrin. The peak observed at 1561 cm−1 may be assigned to the bending vibrations of water molecules trapped into the ZrO2 nano particles.
The XRD analysis of ZrO2 and ZrO2-supported-β-cyclodextrin nanoparticles indicate five characteristic peaks at 2θ = 30.2°, 35.15°, 50.44°, 60.14°, and 62.98° corresponding to (111), (200), (220), (311), and (331) planes, respectively as shown in Fig. 2. All diffraction peaks and positions match with those from the JCPDS card (Joint Committee on Powder Diffraction Standards no. 37-1484) calculated from the Scherer's equation
The SEM and TEM of the prepared ZrO2-β-CD nanoparticles were shown in Fig. 3. The TEM image of the catalyst shows that nano particles are highly aggregated. The average size of these particles is about 1–2 nm, which shows a close agreement with the values calculated by XRD data. The SEM image of the supported catalyst confirms that these nanoparticles are uneven-sized particles due to deposition of β-cyclodextrin complex nanoparticles on the surface of ZrO2 and most of them are almost spherical in shape.
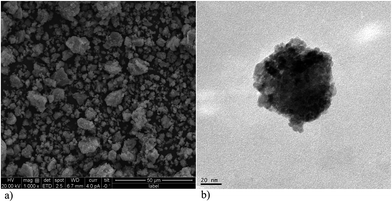 | ||
| Fig. 3 (a) SEM image of ZrO2-supported-β-cyclodextrin; (b) TEM image of ZrO2-supported-β-cyclodextrin. | ||
The at% peaks of the elements were found to be C (39.15%), O (25.04%) and Zr (35.81%) in the EDAX spectrum of ZrO2-β-CD nanoparticles as shown in Fig. 4.
To optimize the reaction conditions for the synthesis of 2,4,5-trisubstituted imidazoles, benzaldehyde, benzil and ammonium acetate were used as model substrates and the reaction was screened in different solvents, temperatures, and various amounts of catalyst and the results are summarized in Table 1. In the presence of β-CD and water as solvent at 80 °C, reaction yielded the desired product 3a in 30% yield (Table 1, entry 1), the low yield may be due to the partial misicibility of benzil and aldehyde in aqueous solvent at 80 °C. Next, a series of catalysts were examined, among them ZrO2-β-CD was found to be better catalyst for the formation of 3a with 65% yield in presence of water as solvent at 100 °C (Table 1, entry 5). We next examined the reaction in presence of various solvents and solvent free conditions (Table 1, entries 6–16). In the presence of solvents the reaction produced the product 3a in low yields (Table 1 entries 1–8) and under solvent free condition the reaction underwent smoothly and gave the desired product in good yield (Table 1 entries 9–15). Initially 20 mol% ZrO2-β-CD at 100 °C, gave the desired product in very low yield (Table 1, entry 11). When the catalyst load was increased from 20 to 40 mol%, it resulted in increase in yield of 3a up to 96% and the reaction was completed in just 40 minutes at 100 °C (Table 1, entry 13).
| Entry | Catalyst | Catalyst loading (mol%) | Nitrogen source | Solvent | Temp (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: benzil (1.0 mmol), benzaldehyde (1.0 mmol), NH4OAc (2.0 mmol), catalyst and solvent (10 mL), or neat.b Isolated yield. | |||||||
| 1 | β-CD | 40 | NH4OAc | Water | 80 | 7 | 30 |
| 2 | ZrO2–Cu2(II)-β-CD | 40 | NH4OAc | Water | 80 | 6 | 40 |
| 3 | ZrO2–Al2O3 | 40 | NH4OAc | Water | 100 | 6 | 60 |
| 4 | ZrO2-β-CD | 10 | NH4OAc | Water | 100 | 6 | 50 |
| 5 | ZrO2-β-CD | 20 | NH4OAc | Water | 100 | 4 | 65 |
| 6 | ZrO2–Cu2(II)-β-CD | 40 | NH4OAc | DMF | 100 | 8 | 55 |
| 7 | ZrO2-β-CD | 40 | NH4OAc | DMF | 100 | 4 | 60 |
| 8 | ZrO2-β-CD | 40 | NH4OAc | EtOH | 100 | 3 | 60 |
| 9 | β-CD | 40 | NH4OAc | Neat | 100 | 2 | 40 |
| 10 | ZrO2–Al2O3 | 40 | NH4OAc | Neat | 100 | 1 | 50 |
| 11 | ZrO2-β-CD | 20 | NH4OAc | Neat | 100 | 1.3 | 50 |
| 12 | ZrO2–Cu2-β-CD | 20 | NH4OAc | Neat | 100 | 4 | 70 |
| 13 | ZrO2-β-CD | 40 | NH4OAc | Neat | 100 | 0.4 | 96 |
| 14 | ZrO2–Cu2(II)-β-CD | 40 | NH4OAc | Neat | 100 | 4 | 70 |
| 15 | ZrO2–Al2O3 | 40 | NH4OAc | Neat | 100 | 0.5 | 60 |
| 16 | ZrO2-β-CD | 40 | NH4Cl | Neat | 100 | 3 | — |
With the optimized reaction condition in hand, we next explored the generality and scope of the protocol using various aldehydes and keeping 1,2-diketone same for the synthesis of 2,4,5-trisubstituted imidazoles. The results are shown in Table 2. These results show that the reactions are equally facile with both electron-donating and electron-withdrawing substituent's present on the aromatic aldehydes resulting in high yields of the desired imidazoles. The known products were characterized by comparing their physical properties with those reported in the literature.
| Entry | Diketone | R | Product | Time (h) | Yieldb,c (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1,2-diketone (1.0 mmol), aldehyde (1.0 mmol), NH4OAc (2.0 mmol), ZrO2-β-CD (40 mol%), neat.b Isolated yield.c Literature reported compounds. | |||||
| 1 | 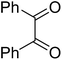 |
 |
 |
0.40 | 96 (ref. 57) |
| 2 | 1 | 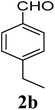 |
 |
0.40 | 85 (ref. 57) |
| 3 | 1 |  |
 |
0.50 | 86 (ref. 58) |
| 4 | 1 |  |
 |
0.50 | 87 (ref. 58) |
| 5 | 1 | 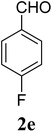 |
 |
0.50 | 89 (ref. 58) |
| 6 | 1 |  |
 |
0.35 | 92 (ref. 58) |
| 7 | 1 |  |
 |
0.45 | 90 (ref. 58) |
| 8 | 1 |  |
 |
0.50 | 97 (ref. 58) |
| 9 | 1 |  |
 |
0.45 | 89 (ref. 58) |
| 10 | 1 |  |
 |
0.35 | 97 (ref. 59) |
| 11 | 1 |  |
 |
0.35 | 96 |
| 12 | 1 |  |
 |
0.40 | 80 (ref. 60) |
| 13 | 1 | 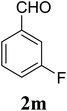 |
 |
0.45 | 94 |
| 14 | 1 |  |
 |
0.55 | 98 |
We next explored the generality of this protocol for the synthesis of 1,2-disubstituted benzimidazoles. Initially, benzaldehyde and 1,2-phenylenediamine were selected as model substrates for the synthesis of 1,2-disubstituted benzimidazoles and the results are presented in Table 3. The reaction in presence of ZrO2-β-CD in EtOH as solvent at room temperature gave desired 1-benzyl-2-phenyl-1H-benzo[d]imidazole 5a in 55% yield (Table 3, entry 1). We next examined the reaction using different catalysts such as ZrO2-β-CD, nano TiO2, nano CuO, Mo/ZrO2, ZnO–Al2O3, β-CD, ZrO2–Al2O3 and ZrO2–Cu2-β-CD (Table 3, entries 1–11). Among them ZrO2-β-CD was found to be better catalyst for the synthesis of 1,2-disubstituted benzimidazoles. We next converged our interest to study the effect of solvents on the product yield. Among various solvent screened, DMF was found to be better and gave the desired product 5a in 85% yield at 100 °C (Table 3, entry 4). Then the reaction was performed using ZrO2-β-CD as catalyst by varying temperature and mol% of catalyst under solvent free condition. Initially 20 mol% of ZrO2-β-CD at 100 °C gave only 85% of yield even after 1.3 h (Table 3, entry 13). Then the reaction was screened by increase in catalyst load from 20 to 40 mol% which gave increase in yield up to 95% (Table 3, entry 14).
| Entry | Catalyst | Mol (%) | Solvent | Temp (°C) | Time (h) | Yieldb |
|---|---|---|---|---|---|---|
| a Reaction conditions: 1,2-phenylenediamine (1.0 mmol), benzaldehyde (2.0 mmol), catalyst and solvent (10 mL), or neat.b Isolated yield, rt = room temperature. | ||||||
| 1 | ZrO2-β-CD | 40 | EtOH | Rt | 12 | 55 |
| 2 | ZrO2-β-CD | 40 | H2O | Rt | 18 | 60 |
| 3 | ZrO2-β-CD | 40 | EtOH | 100 | 2 | 70 |
| 4 | ZrO2-β-CD | 40 | DMF | 100 | 3 | 85 |
| 5 | Nano TiO2 | 40 | DMF | 100 | 8 | 55 |
| 6 | Nano CuO | 40 | DMF | 100 | 12 | 45 |
| 7 | Mo/ZrO2 | 40 | DMF | 100 | 18 | 60 |
| 8 | ZnO–Al2O3 | 40 | DMF | 100 | 5 | 50 |
| 9 | β-CD | 40 | DMF | 100 | 12 | 70 |
| 10 | ZrO2–Al2O3 | 40 | DMF | 100 | 8 | 60 |
| 11 | ZrO2–Cu2-β-CD | 40 | DMF | 100 | 5 | 80 |
| 12 | ZrO2-β-CD | 10 | Neat | 100 | 2.3 | 70 |
| 13 | ZrO2-β-CD | 20 | Neat | 100 | 1.3 | 85 |
| 14 | ZrO2-β-CD | 40 | Neat | 100 | 1 | 95 |
| 15 | Nano TiO2 | 40 | Neat | 100 | 1.15 | 75 |
| 16 | Nano CuO | 40 | Neat | 100 | 2.15 | 40 |
| 17 | β-CD | 40 | Neat | 100 | 1.3 | 60 |
| 18 | ZnO–Al2O3 | 40 | Neat | 100 | 2 | 50 |
After optimization of the reaction condition, different aldehydes and 1,2-phenylenediamine were used as substrates for the synthesis 1,2-disubstituted benzimidazoles, and the results are shown in Table 4. Reaction underwent comparatively fast when electron-donating substituent's such as methyl, methoxy, ethyl, or propyl group were present on the substrates. Whereas, the electron-withdrawing substituent's such as CF3, F, Br, Cl, NO2 took more time for completion of reaction. Structures of the newly synthesized compounds were characterized by spectroscopic and elemental analysis data. The known products were characterized by comparing their physical properties with those reported in the literature.
| Entry | Diamine | Aldehyde | Product | Time (h) | Yieldb,c (%) |
|---|---|---|---|---|---|
| a Reaction conditions: and 1,2-phenylenediamine (1.0 mmol), aldehyde (2.0 mmol), ZrO2-β-CD (40 mol%) neat.b Isolated yield.c Literature reported compounds. | |||||
| 15 |  |
 |
 |
1.00 | 93 (ref. 14) |
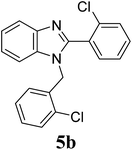
| 16 | 4 | 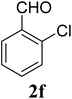 |
 |
1.00 | 85 (ref. 14) |
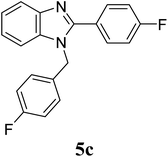
| 17 | 4 |  |
 |
1.50 | 95 (ref. 15) |
| 18 | 4 |  |
 |
1.30 | 87 (ref. 16) |
| 19 | 4 |  |
 |
1.45 | 92 (ref. 16) |
| 20 | 4 |  |
 |
1.30 | 96 (ref. 17) |
| 21 | 4 |  |
 |
2.00 | 80 (ref. 18) |
| 22 | 4 |  |
 |
1.40 | 93 (ref. 19) |
| 23 | 4 | 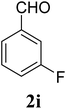 |
 |
1.45 | 88 |
| 24 | 4 | 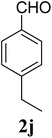 |
 |
1.40 | 90 |
| 25 | 4 |  |
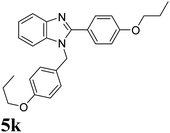 |
1.35 | 90 |
Since many nitrogen containing heterocyclic derivatives61 possess wide range of biological activities we have screened all the synthesized derivatives for their antibacterial activity. The antibacterial activity of the synthesized test samples 3(a–n) and 5(a–k) were screened for their antibacterial activity against both Gram-positive and Gram-negative bacterial strains, using previous reported procedure.62 The results shown in Table 5 indicates that these compounds exhibit a variable zone of inhibition ranging from 4–32 mm. These compounds shown moderate antibacterial activity against Gram-positive bacteria Bacillus subtilis (MTCC 121), with inhibition zone around <19 mm except for the compounds 3b, 3c, 3n, 5a, 5c, 5e–j, which do not show any antibacterial activity. In contrast compounds 3a–n, 5a–c and 5g–j did not inhibit the growth of Klebsiella pneumoniae, Pseudomonas aeruginosa, Enterobacter aeruginosa, Shigella flexneri (except compounds 3f, 3g, 3h in Pseudomonas aeruginosa) even at maximum concentration of 10 μg per disc. However the compound 5f showed very good inhibition to the growth of Klebsiella pneumonia and Enterobacter aeruginosa with inhibition value of 32 and 26 mm respectively.
| Comp | R | Zone of inhibitiona (mm), dose (5 μg, 10 μg per disc) | ||||||
|---|---|---|---|---|---|---|---|---|
| μg per disc | K.pb | B.sc | P.ad | E.ae | S.ff | S.tg | ||
| a The values indicate the average diameters in mm (of two trials) for the zone of growth inhibition observed after 24 h of incubation at 37 °C.b Klebsiella pneumoniae MTCC 7407.c Bacillus subtilis MTCC 121.d Pseudomonas aeruginosa MTCC 7903.e Enterobacter aeruginosa MTCC 7325.f Shigella flexneri MTCC 1457.g Salmonella typhi MTCC 733. | ||||||||
| 3a | C6H5 | 5 | — | 5 | — | — | — | 5 |
| 10 | — | 13 | — | — | — | 10 | ||
| 3b | 4-EtC6H4 | 5 | — | — | — | — | — | — |
| 10 | — | — | — | — | — | — | ||
| 3c | 4-CF3C6H4 | 5 | — | — | — | — | — | — |
| 10 | — | — | 10 | — | — | — | ||
| 3d | C7H5O2 | 5 | — | 5 | — | — | — | — |
| 10 | — | 13 | — | — | — | — | ||
| 3e | 4-FC6H4 | 5 | — | 8 | — | — | — | — |
| 10 | — | 18 | — | — | — | 10 | ||
| 3f | 4-OMeC6H4 | 5 | — | 9 | 5 | — | — | — |
| 10 | — | 18 | 12 | — | — | — | ||
| 3g | 4-MeC6H4 | 5 | — | 9 | 6 | — | — | — |
| 10 | — | 18 | 13 | — | — | — | ||
| 3h | 2-ClC6H4 | 5 | — | 8 | 6 | — | — | — |
| 10 | — | 19 | 15 | — | — | — | ||
| 3i | 3-NO2C6H4 | 5 | — | 7 | — | — | — | — |
| 10 | 8 | 15 | — | — | — | — | ||
| 3j | 3,4-DiOMeC6H3 | 5 | — | 8 | — | — | — | — |
| 10 | — | 17 | — | — | — | — | ||
| 3k | 2,3-DiOMeC6H3 | 5 | — | 5 | — | — | — | — |
| 10 | — | 13 | — | — | — | — | ||
| 3l | 3-BrC6H4 | 5 | — | 5 | — | — | — | — |
| 10 | — | 11 | — | — | — | |||
| 3m | 3-FC6H4 | 5 | — | 6 | — | — | — | — |
| 10 | — | 13 | — | — | — | 10 | ||
| 3n | 4-PropC6H4 | 5 | — | — | 10 | — | — | — |
| 10 | — | — | — | — | — | — | ||
| 5a | C6H5 | 5 | — | — | — | — | 5 | — |
| 10 | — | — | — | — | 12 | 18 | ||
| 5b | 2-ClC6H4 | 5 | — | 7 | — | — | 8 | 7 |
| 10 | — | 14 | 20 | — | 13 | 15 | ||
| 5c | 4-FC6H4 | 5 | — | — | — | — | 4 | 7 |
| 10 | — | — | — | — | 12 | 15 | ||
| 5d | 4-OMeC6H4 | 5 | 10 | — | — | 13 | 7 | 6 |
| 10 | 15 | 10 | — | 26 | 10 | 14 | ||
| 5e | 4-MeC6H4 | 5 | 8 | — | — | 14 | 6 | 5 |
| 10 | 13 | — | — | 26 | 10 | 11 | ||
| 5f | C7H5O2 | 5 | 15 | — | — | 13 | 7 | 4 |
| 10 | 32 | — | — | 26 | 12 | 13 | ||
| 5g | 3-NO2C6H4 | 5 | — | — | — | — | — | 8 |
| 10 | — | — | 17 | — | 9 | 16 | ||
| 5h | 3-BrC6H4 | 5 | — | — | — | — | — | — |
| 10 | — | — | — | — | — | — | ||
| 5i | 3-FC6H4 | 5 | — | — | — | — | — | — |
| 10 | — | — | — | — | — | — | ||
| 5j | 4-EtC6H4 | 5 | — | 8 | — | — | 8 | — |
| 10 | — | 15 | — | — | 16 | 13 | ||
| 5k | 4-PropC6H4 | 5 | 5 | — | — | — | 5 | — |
| 10 | 10 | — | — | — | 12 | 9 | ||
| Streptomycin | 10 | 22 | 24 | 23 | 18 | 20 | 17 | |
Conclusion
In conclusion, we have developed an efficient and safe protocols for the synthesis of 2,4,5-trisubstituted imidazoles and 1,2-disubstituted benzimidazoles under solvent free conditions. More efficient nanoparticle-catalyst ZrO2-supported-β-cyclodextrin (1–5 nm range) was easily prepared by co-precipitation method. The analysis data reveal that the nano ZrO2-supported-β-cyclodextrin was an effective heterogeneous reusable catalyst for the synthesis of 2,4,5-trisubstituted imidazoles and 1,2-disubstituted benzimidazoles under solvent-free mild conditions. Further the compounds have been screened for the antibacterial activity. Compound 5f at 10 μg per disc showed potent inhibitory activity against K. pneumoniae, compared to the standard (streptomycin 10 μg per disc) at the same concentration. None the less, further studies needed to be conducted for molecular mechanism of 5f derivatives against the human pathogen K. pneumonia. The present study revealed the potential application and development of synthetic novel molecules against human pathogen and it also proposed for some more studies relating to antimicrobial during further studies.Experimental section
General methods
Zirconium oxychloride (ZrOCl2·8H2O, >99.0%), ammonia (NH3·H2O, 25%) was purchased from s.d. Fine-Chem Ltd, India. β-Cyclodextrin was purchased from Sigma-Aldrich India. Deionized water was used for all workup procedures. Melting points were measured on secor INDIA apparatus and are uncorrected. 1H NMR and 13C NMR were recorded on VNMRS-400 (Agilent Technologies) NMR spectrometer in CDCl3. Tetramethylsilane (TMS; δ = 0.00 ppm) served as internal standard. The corresponding residual non-deuterated solvent signal (CDCl3: δ = 77.00 ppm) was used as internal standard for 13C NMR. Performed column chromatography on silica gel 60–120 mesh (Merck). Unless otherwise noted, materials obtained from commercial suppliers were used without further purification. Mass spectra were measured with Micromass Q-Tof (HRESI-MS). DMF was dried over CaH2 for 2 h and filtered. The catalyst was characterized by ATR-Fourier transform infrared (ATR-IR) spectra, recorded using a Thermo-Nicolet FTIR spectrometer (Model 5700, Madison, WI) fitted with a single bounce attenuated total reflectance (ATR) accessory with a ZnSe crystal of range 400–4000 cm−1. X-ray powder diffraction (PXRD) was carried out on a XRD 7000, Shimandzu diffractometer with CuKα radiation. Scanning electron microscopy (SEM) images were obtained using a JEOL JXA-8530F microscope. Transmission electron microscopy (TEM) images were performed on PHILIPS CM200 electron microscope at an acceleration voltage of 20–200 kV. TGA thermograms of the nanoparticles were obtained under nitrogen on a Perkin-Elmer TGA 7 analyzer at a heating rate of 20 °C min−1.Preparation of ZrO2-supported-β-cyclodextrin nanoparticles
The ZrO2-supported-β-cyclodextrin nanoparticles were prepared by a chemical co-precipitation method.25 2.42 g of zirconium oxychloride (ZrOCl2·8H2O) and 1.04 g of-β-cyclodextrin were dissolved in 50 mL of deionized water under intense stirring at 90 °C. Subsequently, ammonia (25%) was added drop wise to the reaction mixture with stirring. The nanoparticles formed were collected by filtration, washed with distilled water repeatedly, and dried for 24 h at room temperature in a high vacuum.General procedure for the synthesis of 2,4,5-trisubstituted imidazole derivatives or 2,5-disubstituted benzimidazole derivatives in the presence of ZrO2-supported-β-cyclodextrin nanoparticles as catalyst under solvent free conditions
To a mixture, of benzil (1 mmol), benzaldehyde (1 mmol), and NH4OAc (2 mmol) for 2,4,5-trisubstituted imidazole derivatives or to a mixture of o-phenylenediamine (1 mmol) and aldehyde (2 mmol) for 1,2-disubsubstituted benzimidazole derivatives, was added ZrO2-β-Cd (40 mol%). The resulting mixture was heated at 100 °C on an oil bath under neat conditions for appropriate time till the completion of the reaction. After completion of reaction, as monitored by TLC (eluent: petroleum ether: ethyl acetate: 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), the reaction was cooled to room temperature. The solid thus formed was dissolved in acetone and the catalyst separated by filtration. The mixture was concentrated on a rotary evaporator under reduced pressure and the product obtained was washed with water and recrystallized from acetone–water 9
3), the reaction was cooled to room temperature. The solid thus formed was dissolved in acetone and the catalyst separated by filtration. The mixture was concentrated on a rotary evaporator under reduced pressure and the product obtained was washed with water and recrystallized from acetone–water 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) to offer the pure 2,4,5-trisubstituted imidazole derivatives.
1 (v/v) to offer the pure 2,4,5-trisubstituted imidazole derivatives.
Reusability studies
Recovery of the nano-ZrO2-supported-β-cyclodextrin catalyst was easy and efficient. When the reaction was complete, the precipitated products were dissolved by acetone and nano-ZrO2-supported-β-cyclodextrin was separated by centrifugation followed by decantation (ethanol, 5 mL). The isolated solid phase (nano-ZrO2-supported-β-cyclodextrin) was then dried under reduced pressure and reused for four runs without any appreciable loss in the product yield and its catalytic activity. The same results were obtained for the catalyst recovery in condensation of o-phenylenediamine (1 mmol) and benzaldehyde (2 mmol) for synthesis of the corresponding 1,2-disubstituted benzimidazole under solvent-free conditions. Therefore, the methodologies are environmentally friendly, cost effective and industrially important because of the catalyst reuse and the use of safe reaction media.Acknowledgements
YRG is thankful to UGC-BSR for meritorious fellowship Order no. DV-5/662/RFSMS/2012–13, New Delhi, India for financial support and we are also thankful to IOE, University of Mysore and NCBS, Bengaluru, Karnataka, India for their support in carrying out research work regarding the single crystal XRD and TEM analysis.References
- (a) C. Guo, C. Zhang, X. Li, W. Li, Z. Xu, L. Bao, Y. Ding, L. Wang and S. Li, Bioorg. Med. Chem. Lett., 2013, 23, 5850 CrossRef CAS PubMed; (b) S. Hack, B. Wörlein, G. Höfner, J. Pabel and K. T. Wanner, Eur. J. Med. Chem., 2011, 46, 1483 CrossRef CAS PubMed; (c) J. G. Lombardino and E. H. Wiseman, J. Med. Chem., 1974, 17, 1182 CrossRef CAS; (d) X. Q. Wang, L. X. Liu, Y. Li, C. J. Sun, W. Chen, L. Li, H. B. Zhang and X. D. Yang, Eur. J. Med. Chem., 2013, 62, 111 CrossRef CAS PubMed; (e) M. Mccann, R. Curran, M. Ben-Shoshan, V. McKee, M. Devereux, K. Kavanagh and A. Kellett, Polyhedron, 2013, 56, 180 CrossRef CAS PubMed; (f) H. Nabata, M. Uchino, A. Okazaki, T. Yamazaki and K. Sakai, Jpn. J. Pharmacol., 1987, 44, 93 CrossRef CAS; (g) J. Liu, J. Qiu, M. Wang, L. Wang, L. Su, J. Gao, Q. Gu, J. Xu, S. L. Huang, L. Q. Gu, Z. S. Huang and D. Li, Biochim. Biophys. Acta, 2014, 1840, 2886 CrossRef CAS PubMed; (h) J. Kang, L. Han, Z. Chen, J. Shen, J. Nan and Y. Zhang, Food Chem., 2014, 159, 445 CrossRef CAS PubMed.
- J. Kulhánek and F. Bureš, Beilstein J. Org. Chem., 2012, 8, 25 CrossRef PubMed.
- C. Yao-Te, H. So-Lin, C. Guan-Yu, S. Ming-Hsin, A. S. Thounaojam, D. E. Wei-Guang and W. Kung-Hwa, Adv. Funct. Mater., 2008, 18, 2356 CrossRef PubMed.
- W. Zhiming, L. Ping, C. Shuming, G. Zhao, S. Fangzhong, Z. Wensi, X. Yuanxiang, K. W. Hoi Sing and M. Yuguang, J. Mater. Chem., 2011, 21, 5451 RSC.
- Y. Zijun and R. B. Elliot, J. Phys. Chem. A, 2013, 117, 1756 Search PubMed.
- B. Milde, D. Schaarschmidt, T. Rüffer and H. Lang, Dalton Trans., 2012, 41, 5377 RSC.
- W. A. Denny, G. W. Rewcastle and B. C. Baguley, J. Med. Chem., 1990, 33, 814 CrossRef CAS.
- T. Fonseca, B. Gigante and T. L. Gilchrist, Tetrahedron, 2001, 57, 1793 CrossRef CAS.
- R.-H. Tan, L.-C. Ding and X.-J. Wei, Chin. J. Med. Chem., 2001, 11, 259 CAS.
- D. Yang, D. Fokas, J. Li, L. Yu and C. M. Baldino, Synthesis, 2005, 1, 47–56 CrossRef.
- Y. Bai, J. Lu, Z. Shi and B. Yang, Synlett, 2001, 11, 544 Search PubMed.
- E. Hasegawa, A. Yoneoka, K. Suzuki, T. Kato, T. Kitazume and K. Yangi, Tetrahedron, 1999, 55, 12957 CrossRef CAS.
- G. A. Molander and K. Ajayi, Org. Lett., 2012, 14, 4242 CrossRef CAS PubMed.
- G. Pranab and M. Amitava, Tetrahedron Lett., 2012, 53, 6483 CrossRef PubMed.
- S. Samuthirarajan and K. Mayilvasagam, Tetrahedron Lett., 2014, 55, 1971 CrossRef PubMed.
- R. Chebolu, D. N. Kommi, D. Kumar, N. Bollineni and A. K. Chakraborti, J. Org. Chem., 2012, 77, 10158 CrossRef CAS PubMed.
- D. Kumar, D. N. Kommi, R. Chebolu, S. K. Garg, R. Kumar and A. K. Chakraborti, RSC Adv., 2013, 3, 91 RSC.
- M. Chakrabarty, R. Mukherjee, S. Karmakar and Y. Harigaya, Monatsh. Chem., 2007, 138, 1279 CrossRef CAS.
- S. Radheshyam, S. Sachin and N. Jayashree, Tetrahedron Lett., 2013, 54, 6986 CrossRef PubMed.
- G. Vikash Kumar, K. Dipratn, C. Amrita and B. Mainak, Tetrahedron Lett., 2013, 54, 5505 CrossRef PubMed.
- M. I. Suleman, K. M. Vinod and K. M. Sisir, Tetrahedron Lett., 2013, 54, 579 CrossRef PubMed.
- H. Debus, Ann. Chem. Pharm., 1858, 107, 199 CrossRef PubMed.
- (a) K. Sivakumar, A. Kathirvel and A. Lalitha, Tetrahedron Lett., 2010, 51, 3018 CrossRef CAS PubMed; (b) S. N. Murthy, B. Madhav and Y. V. D. Nageswar, Tetrahedron Lett., 2010, 51, 5252 CrossRef CAS PubMed; (c) I. M. David, M. Bahramnejad and M. Dabiri, Tetrahedron Lett., 2013, 54, 2591 CrossRef PubMed; (d) S. Maity, S. Pathak and A. Pramanik, Tetrahedron Lett., 2013, 54, 2528 CrossRef CAS PubMed; (e) N. P. Amit and K. A. Devendra, Tetrahedron Lett., 2014, 55, 1835 CrossRef PubMed; (f) Z. Zohre and S. Javad, New J. Chem., 2014, 38, 4555 RSC; (g) S. Javad, A. Zahra and N. Simin, J. Saudi Chem. Soc., 2012 DOI:10.1016/j.jscs.2012.10.012; (h) P. F. Zhang and Z. C. Z. Chen, Synthesis, 2001, 2075 CrossRef CAS.
- S. Tan, X. Li, Y. Guo and Z. Zhang, Nanoscale, 2013, 5, 860 RSC.
- T. Yong, I. Roy, M. T. Swihart and P. N. Prasad, J. Mater. Chem., 2009, 19, 4655 RSC.
- Z. Zarnegar and J. Safari, New J. Chem., 2014, 38, 4555 RSC.
- (a) L. Bao-Le, Z. Mo, H. Hai-Chuan, D. Xia and Z. Zhan-Hui, New J. Chem., 2014, 38, 2435 RSC; (b) D. A. Kotadia, U. H. Patel, S. Gandhi and S. S. Soni, RSC Adv., 2014, 4, 32826 RSC.
- (a) Y. Ding, Y. Wang, L. Zhang, H. Zhang, C. M. Li and Y. Lei, Nanoscale., 2011, 3, 1149 RSC; (b) O. A. Sadik, A. L. Zhou, S. Kikandi, N. Du, Q. Wang and K. Varner, J. Environ. Monit., 2009, 11, 1782 RSC; (c) X. D. Wang, O. S. Wolfbeis and J. R. Meier, Chem. Soc. Rev., 2013, 42, 7834 RSC.
- D. Jariwala, V. K. Sangwan, L. J. Lauhon, T. J. Marks and M. C. Hersam, Chem. Soc. Rev., 2013, 42, 2824 RSC.
- J. Liao, Z. Yang, H. Wu, D. Yan, J. Qiu, Z. Song, Y. Yang, D. Zhou, Z. Yin, H. Kang, Y. Zhu, X. Yang, J. Shen, C. Chen and C. Li, J. Mater. Chem. C, 2013, 1, 6541 RSC.
- H. Kang, Y. Zhu, X. Yang, J. Shen, C. Chen and C. Li, New J. Chem., 2010, 34, 2166 RSC.
- R. Narayanan and M. A. El-Sayed, J. Phys. Chem. B, 2005, 109, 12663 CrossRef CAS PubMed.
- (a) J. Zhang and P. X. Ma, Adv. Drug Delivery Rev., 2013, 65, 1215 CrossRef CAS PubMed; (b) C. Tudisco, V. Oliveri, M. Cantarella, G. Vecchio and G. G. Condorelli, Eur. J. Inorg. Chem., 2012, 5323 CrossRef CAS PubMed.
- B. Aswathy, G. S. Avadhani, S. Suji and G. Sony, Front. Mater. Sci., 2012, 6, 168 CrossRef.
- K. Cai, J. Li, Z. Luo, Y. Hu, Y. Hou and X. Ding, Chem. Commun., 2011, 47, 7719 RSC.
- R. Freeman, T. Finder, L. Bahshi and I. Willner, Nano Lett., 2009, 9, 2073 CrossRef CAS PubMed.
- (a) B. Madhav, S. N. Murthy, V. P. Reddy, K. Rama Rao and Y. V. D. Nageswar, Tetrahedron Lett., 2009, 50, 6025 CrossRef CAS PubMed; (b) K. Ramesh, K. Karnakar, G. Satish, B. S. P. Anil Kumar and Y. V. D. Nageswar, Tetrahedron Lett., 2012, 53, 6936 CrossRef CAS PubMed; (c) D. R. Patil, Y. B. Wagh, P. G. Ingole, K. Singh and D. S. Dalal, New J. Chem., 2013, 37, 3261 RSC; (d) B. Kaboudin, R. Mostafalu and T. Yokomatsu, Green Chem., 2013, 15, 2266 RSC.
- K. Tanabe, Mater. Chem. Phys., 1985, 13, 347 CrossRef CAS.
- R. C. Garvie, R. H. Hannink and R. T. Pascoe, Nature, 1975, 258, 703 CrossRef CAS PubMed.
- G. D. Wilk, R. M. Wallace and J. M. Anthony, J. Appl. Phys., 2001, 89, 5243 CrossRef CAS PubMed.
- G. Y. Guo and Y. L. Chen, J. Mater. Chem., 2001, 11, 1283 RSC.
- S. F. Yin and B. Q. Xu, ChemPhysChem, 2003, 4, 277 CrossRef CAS PubMed.
- A. H. Heuer and L. W. Hobbs, Science and technology of zirconia, American Ceramic Society, Columbus and Ohio, 1981, vol. 3 Search PubMed.
- M. Inoue, H. Kominami and T. Inui, Appl. Catal., A, 1993, 97, 25 CrossRef.
- J. Joo, T. Yu, Y. W. Kim, H. M. Park, F. Wu, J. Z. Zhang and T. Hyeon, J. Am. Chem. Soc., 2003, 125, 6553 CrossRef CAS PubMed.
- R. C. Buchanan and S. Pope, J. Electrochem. Soc., 1983, 130, 962 CrossRef CAS PubMed.
- A. Krell, J. Klimke and T. Hutzler, Opt. Mater., 2009, 31, 1144 CrossRef CAS PubMed.
- J. Robertson, Rep. Prog. Phys., 2006, 69, 327 CrossRef CAS.
- G. Liu and Y. Lin, Anal. Chem., 2005, 77, 5894 CrossRef CAS PubMed.
- S. Liu and M. Y. Han, Chem.–Asian J., 2010, 5, 36 CAS.
- J. Lu, J. B. Zang, S. X. Shan, H. Huang and Y. H. Wang, Nano Lett., 2008, 8, 4070 CrossRef CAS PubMed.
- T. Z. Sholklapper, V. Radmilovic, C. P. Jacobson, S. J. Isco and L. C. De Jonghe, Electrochem. Solid-State Lett., 2007, 10, 74 CrossRef PubMed.
- X. Luo, A. Morrin, A. J. Killard and M. R. Smyth, Electroanalysis, 2006, 18, 319 CrossRef CAS PubMed.
- S. A. Steiner, Th. F. Baumann, B. C. Bayer, R. Blume, M. A. Worsley, W. J. Moberly Chan, E. L. Shaw, R. Schlogl, A. J. Hart, S. Hofmann and B. L. Wardle, J. Am. Chem. Soc., 2009, 131, 12144 CrossRef CAS PubMed.
- (a) Y. R. Girish, K. S. S. Kumar, H. S. Manasa and S. Shashikanth, J. Chin. Chem. Soc., 2014, 61, 1175 CrossRef CAS PubMed; (b) Y. R. Girish, K. S. S. Kumar, U. Muddegowda, N. K. Lokanath, K. S. Rangappa and S. Shashikanth, RSC Adv., 2014, 4, 55800 RSC; (c) Y. R. Girish, K. R. Raghavendra, D. Nagaraja, K. S. S. Kumar and S. Shashikanth, Chin. J. Chem., 2015, 33, 181 CrossRef CAS PubMed.
- L. Bai, A. F. Wyrwalski, C. Machut, P. Roussel, E. Monflier and A. Ponchel, CrystEngComm, 2013, 15, 2076 RSC.
- K. Ramesh, S. Narayana Murthy, K. Karnakar, Y. V. D. Nageswar, K. Vijayalakhshmi, B. L. A. P. Devi and R. B. N. Prasad, Tetrahedron Lett., 2012, 53, 1126 CrossRef CAS PubMed.
- X. Min and L. Yue-dong, J. Mol. Catal. A: Chem., 2007, 265, 205 CrossRef PubMed.
- S. Saikat Das, H. Parasa and K. Dilip, Tetrahedron Lett., 2008, 49, 2216 CrossRef PubMed.
- M. Jafarpour, A. Rezaeifard, M. Ghahramaninezhad and T. Tabibi, New J. Chem., 2013, 37, 2087 RSC.
- (a) V. Srinivas, C. D. Mohan, C. P. Baburajeev, S. Rangappa, S. Jagadish, J. E. Fuchs, A. Y. Sukhorukov, Chandra, D. J. Mason, K. S. Sharath Kumar, M. Madegowda, A. Bender, Basappa and K. S. Rangappa, Bioorg. Med. Chem. Lett., 2015, 25, 2931 CrossRef CAS PubMed; (b) K. S. Sharath Kumar, A. Hanumappa, M. Hegde, K. H. Narasimhamurthy, S. C. Raghavan and K. S. Rangappa, Eur. J. Med. Chem., 2014, 81, 341 CrossRef CAS PubMed; (c) K. S. S. Kumar, A. Hanumappa, M. Vetrivel, M. Hegde, Y. R. Girish, T. R. Byregowda, S. Rao, S. C. Raghavan and K. S. Rangappa, Bioorg. Med. Chem. Lett., 2015, 25, 3616 CrossRef PubMed.
- P. Bandyopadhya, M. Sathe, S. Ponmariappan, S. Sharma, P. Sharma, A. K. Srivastava and M. P. Kaushik, Bioorg. Med. Chem. Lett., 2011, 21, 7306 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra13891d |
| This journal is © The Royal Society of Chemistry 2015 |