SO3H and NH2+ functional carbon-based solid acid catalyzed transesterification and biodiesel production†
Abstract
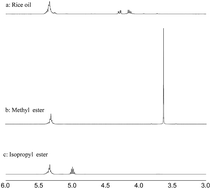
A SO3H and NH2+ functional carbon-based solid acid was used as a highly active heterogeneous catalyst for the transesterification of various carboxylic methyl esters with alcohols under mild conditions. It also showed high catalytic performance for transesterification of triolein with methanol or isopropanol. Furthermore, it was able to catalyze simultaneous esterification and transesterification of rice oil and butter respectively, the yields of biodiesel obtained were up to 94%, and the catalyst could be easily recovered and reused more than ten times without loss of activity, which indicated the carbon-based solid acid was a potential catalyst for the biodiesel industry.

 Please wait while we load your content...
Please wait while we load your content...