Enhanced corneal permeation of coumarin-6 using nanoliposomes containing dipotassium glycyrrhizinate: in vitro mechanism and in vivo permeation evaluation
Abstract
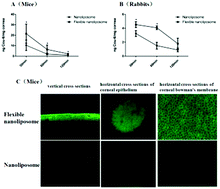
The objective of the present study was to investigate the elasticity of the lipid bilayer of nanoliposomes regarding in vitro cellular uptake/mechanics and in vivo corneal permeation through ocular topical routes. Flexible nanoliposomes, using dipotassium glycyrrhizinate as an edge activator, and their physical properties, membrane elasticity, cellular uptake characterizations and mechanisms, as well as in vivo corneal permeation using rabbits and mice as experimental animals, were investigated and compared with the conventional liposomal formulation composed of soybean phosphatidylcholine and cholesterol. Flexible nanoliposomes required less energy to prepare and had elastic lipid membranes. Compared with nanoliposomes, flexible nanoliposomes showed a significantly higher cellular uptake of coumarin-6. Moreover and interestingly, the flexible nanoliposomes showed different cellular uptake mechanisms in cells. Flexible nanoliposomes also showed significantly higher corneal penetrating ability in in vivo testing. Therefore, the fluidity of the liposomal membrane differently affected cellular uptake/internalization and in vivo corneal penetration of the nanoliposomes, and flexible nanoliposomes might be a promising therapeutic tool for the treatment of ocular surface disorders.

 Please wait while we load your content...
Please wait while we load your content...