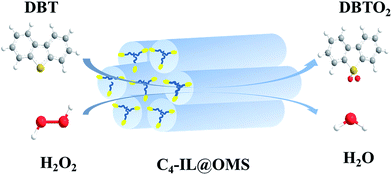
One-pot synthesis of ordered mesoporous silica encapsulated polyoxometalate-based ionic liquids induced efficient desulfurization of organosulfur in fuel†
Ming Zhanga,
Meng Lib,
Qi Chenc,
Wenshuai Zhu*b,
Hongping Lib,
Sheng Yinb,
Yanan Lib and
Huaming Li*ab
aInstitute for Energy Research, Jiangsu University, Zhenjiang 212013, P. R. China. E-mail: lhm@ujs.edu.cn; Fax: +86-511-88791708; Tel: +86-511-88791800
bSchool of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang 212013, P. R. China. E-mail: zhuws@ujs.edu.cn
cSchool of Energy and Power Engineering, Jiangsu University, Zhenjiang 212013, P. R. China
First published on 28th August 2015
Abstract
In this work, a novel hybrid material of an ordered mesoporous silica (OMS) encapsulated polyoxometalate-based ionic liquid [C4mim]3PW12O40 (C4-IL) was successfully fabricated through a one-pot hydrothermal process, and firstly employed in the oxidative desulfurization of organosulfur. The as-prepared material OMS encapsulated C4-IL (C4-IL@OMS) was systematically characterized by Fourier transform infrared, Raman, X-ray diffraction, X-ray photoelectron spectroscopy, nitrogen absorption–desorption isotherms, scanning electron microscopy and transmission electron microscopy. The designed material exhibited robust activity for removing sulfur-containing compounds under mild conditions due to the high dispersion of C4-IL in OMS and lager specific surface area of the catalyst. 31P nuclear magnetic resonance and ultraviolet-visible spectrum were employed to evaluate the stability of the catalyst after the reaction. Additionally, the sulfur removal of the hybrid material C4-IL@OMS could still reach 93% after recycling seven times without obvious decrease in activity.
1. Introduction
Nowadays, removal of sulfur-containing compounds in fuels is considered as one of the most important and challenging procedures in the petroleum industry. The combustion of these sulfur-containing compounds will bring large amounts of SOx, which is a major source of air pollution.1–3 At present, hydrodesulfurization (HDS) is widely applied in removing sulfur-containing compounds around the world. However, aromatic sulfur compounds, such as dibenzothiophene (DBT) and its derivatives were hardly removed by HDS due to their steric hindrance.4,5 Therefore, considerable attentions have been directed to create other low-cost alternatives to reach ultra-desulfurization under mild conditions, such as adsorption,6–8 oxidization,9–14 extraction15,16 and photocatalysis, etc.17–19 In particular, oxidative desulfurization (ODS) is regarded as one of the most promising ways to achieve deep desulfurization since aromatic sulfides could be easily removed under moderate conditions.Polyoxometalates (POMs) have been widely used as active species in various organic transformations because of their unique physical and chemical properties, such as thermal stability, acidic and good redox properties.20 Recently, to enlarge the scope of the application of polyoxometalates, POMs-based catalysts coupled with ionic liquids were developed and exhibited excellent catalytic activity upon epoxidation of olefins,21 selective adsorption of dyes,22 and rearrangement reactions,23 etc. However, owing to negative dissolution of POMs coupled with inorganic/organic counterparts in the common solvent, the extensive applications in various reactions were limited.
In recent years, ionic liquids (ILs) as a novel “green solvent” and catalyst, have attracted wide attention owing to their unique properties, such as good thermal stability, wide liquid range and easy to design.24 Based on these advantages, the catalysts could be dissolved into the ILs phase to provide a liquid–liquid biphasic system, which has been successfully applied in the esterification reaction,25 the dehydration of fructose,26 and oxidation of sulfides.27,28 However, high dosage of ILs is required, and the separation of catalysts used is difficult in these processes, inhibiting their further application in organic reactions. Hence, the heterogenization of homogeneous ILs provided a better way to tackle these limitations. For this purpose, various attempts have been paid to fabricate “supported ionic liquid catalysts” (SILCs) that fulfill these requirements through the immobilization of homogeneous ILs on suitable host, such as silica materials,29–31 polymer,32 MOF7,33,34 and CNT,35 etc.
Compared with the nonporous silica gel originated from the hydrolysis of silica precursors, ordered mesoporous silica materials have attracted great interest in catalysis,36 drug delivery,37 and adsorption,38 owing to its relative large surface area, adjustable pore size, nontoxic and large pore volume. To fully utilize the advantages of the mesoporous silica, considerable efforts have been devoted to prepare mesoporous silica-supported catalyst through various methods. Impregnation and grafting method were widely applied in the synthetic process of SILCs. However, the surface area of support decreased remarkably after the introduction of ILs, and the leaching of active components was serious during reaction.39,40 To overcome these limitations, ILs with some functional groups was introduced into the matrix of the supports by the grafting method, which could improve the stability of ILs in the reaction process.41,42 Unfortunately, the properties and the freedom of ILs may be confined. Hence, the encapsulation of ILs into the architecture of support by one-pot hydrothermal process is of great interest to prepare the SILCs.33,43 The as-synthesized heterogeneous materials could not only reduce the amount of ILs used and increase the stability of ILs, but also benefit for the separation of catalysts after reaction. Besides, these materials could also possess high surface area by removing the templates in the subsequent treatment. Inspired by these advantages, the SILCs achieved from the encapsulation of ILs have been explored in various reactions and exhibited excellent catalytic performance in comparison to the pure ILs and the heterogeneous catalysts obtained from the impregnation and grafting method.
Herein, polyoxometalate-based ionic liquids ([Cxmim]3PW12O40, x = 4, 8, and 16) were successfully encapsulated into the ordered mesoporous silica (OMS) matrix by a facile one-pot hydrothermal procedure (Scheme 1). The designed ordered mesoporous silica encapsulated POMs-based IL catalyst [C4mim]3PW12O40@OMS exhibited excellent activity, high stability and good recyclability in the removal of DBT with an ultra-low dosage of oxidant, and no organic solvent was added as the extractant. The stability of the as-synthesized hybrid material was investigated by 31P NMR and UV-vis analysis. In addition, the possible desulfurization mechanism was evaluated by GC-MS analysis.
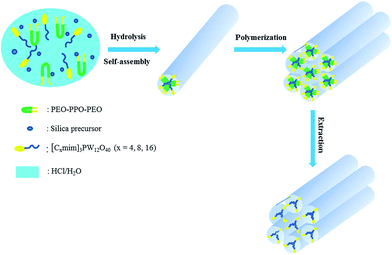 | ||
| Scheme 1 Illustration of the preparation procedure of ordered mesoporous silica encapsulated POMs-based IL. | ||
2. Experimental section
2.1. Materials
Benzothiophene (BT), 3-methylbenzothiphene (3-MBT), dibenzothiophene (DBT), 4-methyldibenzothiophene (4-MDBT) and 4,6-dimethyldibenzothiphene (4,6-DMDBT) were purchased from Aladdin Chemical Co., Ltd. Pluronic P123 (EO20PO70EO20, Mavg = 5800) was supplied by Sigma-Aldrich. 1-Butyl-3-methylimidazole chloride ([C4mim]Cl), 1-octyl-3-methylimidazole chloride ([C8mim]Cl), 1-cetyl-3-methylimidazole chloride ([C16mim]Cl) were obtained from Shanghai Chenjie Chemical Co., Ltd. Phosphotungstic acid (H3PW12O40·14H2O, AR grade), hydrogen peroxide (H2O2, 30 wt%, AR grade), acetonitrile (CH3CN, AR grade), tetraethyl orthosilicate (TEOS) and n-octane (AR grade) were marketed from Sinopharm Chemical Reagents Co., Ltd.2.2. Sample preparation
A series of POMs-based ionic liquids [Cxmim]3PW12O40 (Cx-ILs, x = 4, 8, 16) were prepared according to the study reported,44 and ordered mesoporous silica encapsulated Cx-ILs were synthesized according to the previous report with modification.45 In a typical procedure, 2.668 g of P123 was dissolved in 63 g of HCl solution (1.9 M). After the surfactant was completely dissolved, 4 mL of acetonitrile containing 0.01 mmol of C4-IL was dropped into the above clear solution. Then, 4 mL of TEOS was gently added to the above solution under vigorous stirring for 24 h. The resultant was transferred to an autoclave and hydrothermally treated at 100 °C for 24 h. Then, the obtained products were separated and washed with deionized water and ethanol for three times and dried at 80 °C overnight. Finally, the mesoporous silica was obtained by removing template using ethanol for 72 h, and named as C4-IL@OMS. The obtained sample was with a Si/W molar ratio of 15 in the gel. The ordered mesoporous silica encapsulated POMs-based ILs [C8mim]3PW12O40 and [C16mim]3PW12O40 were prepared with the same Si/W molar ratio through a similar method, and named as C8-IL@OMS and C16-IL@OMS respectively.2.3. Characterization
Fourier transform infrared (FTIR) spectra of as-synthesis material were recorded with a Nicolet FTIR apparatus (Nexus 470, Thermo Electron Corporation) using KBr pellets. Raman spectroscopy was carried out on a DXR Raman spectrometer using a 532 nm excitation. Wide-angle X-ray diffraction (XRD) of samples range from 10 to 80° was collected on a Bruker D8 X-ray diffractometer equipped with Cu Kα radiation. Diffuse reflectance spectra (DSR) were performed on a UV-vis spectrometer (UV-2450, Shimadzu). Small-angle X-ray scattering (SAXS) patterns were obtained using a Nanostar U small-angle X-ray scattering system (Bruker, Germany) equipped with Cu Kα radiation. X-ray photoelectron spectroscopy (XPS) was collected on a PHI5300 with Mg Kα source. Nitrogen absorption–desorption isotherms were measured using a TriStar II 3020 surface area and porosity analyzer (USA). Transmission electron microscopy (TEM) measurements of samples were recorded with a JEM 2010 (Japan) operated at 200 kV. 31P MAS NMR spectra were collected on a Bruker AVANCE III 400WB with 8 kHz.2.4. Catalytic test
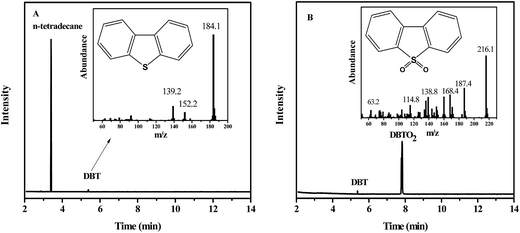
A desired amount of BT, 3-MBT, DBT, 4-MDBT and 4,6-DMDBT was dissolved in n-octane to yield model oil with sulfur content of 250, 500, 500, 500 and 250 ppm, respectively. Desulfurization performance of catalyst was employed in a two-necked flask coupled with thermostatic water bath. In a typical run, 5 mL of model oil was firstly mixed with the catalyst, and then desired amount of H2O2 was injected under stirring. The content of sulfur-containing left in upper oil phase was monitored by the gas chromatography (GC7890A, Agilent). Gas chromatography-mass spectrometry (GC-MS7890/5975C-GC/MSD, Agilent) was used to identify the product of catalytic oxidation reaction.3. Results and discussion
3.1. Characterization of selected samples
FTIR spectra of the pure POMs-based ILs and the OMS encapsulated POMs-based ILs were shown in Fig. 1. For the neat POMs-based ILs (Fig. 1a–c), the characteristic bands of the Keggin units at 1081, 980, 898 and 810 cm−1 could be obviously observed, which were attributed to the P–Oa (central oxygen), W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (terminal oxygen), W–O–W (corner-sharing oxygen) and W–O–W (edge-sharing oxygen) respectively.46,47 The absorption peaks around 1500 cm−1 could be ascribed to the stretching vibration of the imidalium ring. Meanwhile, the peaks in the region of 2900–3100 cm−1 were caused by the vibration of the C–H bond.48 FTIR spectra of the OMS encapsulated POMs-based ILs was displayed in Fig. 1d–f. The bands of Keggin units could also be found. However, the bands at 1081 and 810 cm−1 could not be detected due to the effect of the vibration of the Si–O–Si. In addition, the characteristic peaks of νas(W
O (terminal oxygen), W–O–W (corner-sharing oxygen) and W–O–W (edge-sharing oxygen) respectively.46,47 The absorption peaks around 1500 cm−1 could be ascribed to the stretching vibration of the imidalium ring. Meanwhile, the peaks in the region of 2900–3100 cm−1 were caused by the vibration of the C–H bond.48 FTIR spectra of the OMS encapsulated POMs-based ILs was displayed in Fig. 1d–f. The bands of Keggin units could also be found. However, the bands at 1081 and 810 cm−1 could not be detected due to the effect of the vibration of the Si–O–Si. In addition, the characteristic peaks of νas(W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and νas(W–Oc–W) shifted to 979 and 893 cm−1 respectively, which may arise from the interaction of Keggin units with the host OMS.49,50
O) and νas(W–Oc–W) shifted to 979 and 893 cm−1 respectively, which may arise from the interaction of Keggin units with the host OMS.49,50
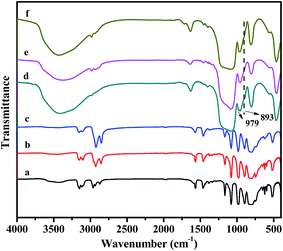 | ||
| Fig. 1 FTIR spectra of (a) C4-IL; (b) C8-IL; (c) C16-IL; (d) C4-IL@OMS; (e) C8-IL@OMS; (f) C16-IL@OMS. | ||
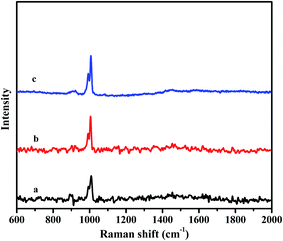
Fig. 2 exhibits the Raman spectra for the synthesized materials. For C4-IL@OMS (Fig. 2a), the characteristic peaks of the Keggin unit at 1008 and 993 cm−1, corresponding to vibration of νs(W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and νas(W
O) and νas(W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), respectively.45,51 As shown in Fig. 2b and c, the vibrations of νs(W–O) and νas(W
O), respectively.45,51 As shown in Fig. 2b and c, the vibrations of νs(W–O) and νas(W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) locating at 1005, 990 cm−1 and 1007, 992 cm−1 were found for C8-IL@OMS and C16-IL@OMS respectively. The small difference about vibration bands of Keggin units for various OMS encapsulated ILs may be assigned to the interaction between the POM anion and the imidazole cation.
O) locating at 1005, 990 cm−1 and 1007, 992 cm−1 were found for C8-IL@OMS and C16-IL@OMS respectively. The small difference about vibration bands of Keggin units for various OMS encapsulated ILs may be assigned to the interaction between the POM anion and the imidazole cation.
The structural information of C4-IL@OMS, C8-IL@OMS and C16-IL@OMS mesoporous catalysts was performed by wide-angle XRD (Fig. 3). For the pure OMS (Fig. 3a), the broad peak around 22° was caused by amorphous silica.52 XRD patterns of different ILs were presented in Fig. 3b–d. It was clearly observed that the diffraction peaks of Keggin unit ranged from 15–35°. However, after the encapsulation of ILs into the OMS structure, the diffraction peaks ascribed to the Keggin anion could not be found, suggesting that the ILs were uniformly dispersed into the OMS matrix.
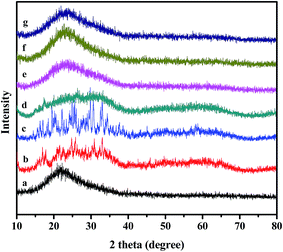 | ||
| Fig. 3 XRD patterns of (a) OMS; (b) C4-IL; (c) C8-IL; (d) C16-IL; (e) C4-IL@OMS; (f) C8-IL@OMS; (g) C16-IL@OMS. | ||
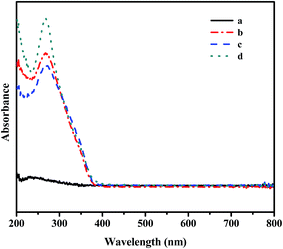
Fig. 4 displays the UV-vis spectra of OMS, C4-IL@OMS, C8-IL@OMS and C16-IL@OMS. For support OMS (Fig. 4a), no obvious absorption peak could be found ranging from 200 to 800 nm. Compared with the OMS, the as-synthesized materials encapsulated ILs exhibited strong peaks around 266 nm (Fig. 4b and c), which was ascribed to the charge transfer from O2− to W6+.53 These results demonstrated that the ILs were successfully encapsulated into the ordered mesoporous silica structure and maintained the structural integrity of Keggin unit, agreeing well with the information from wide-angle XRD analysis.
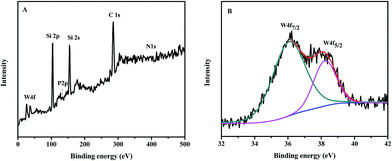
To further study the chemical features of the designed catalyst C4-IL@OMS, XPS spectrum of C4-IL@OMS and W4f XPS analysis were performed (Fig. 5). As shown in Fig. 5A, all elements C, N, P and W for the hybrid material C4-IL@OMS could be found. In addition, Fig. 5B presents peaks of W4f emerged in 36.2 and 38.1 eV were assigned to the WVI of the composite C4-IL@OMS.54,55 These results indicated that the C4-IL remained the structure integrity after encapsulating into the OMS, which was well consistent with the results of UV-vis analysis.
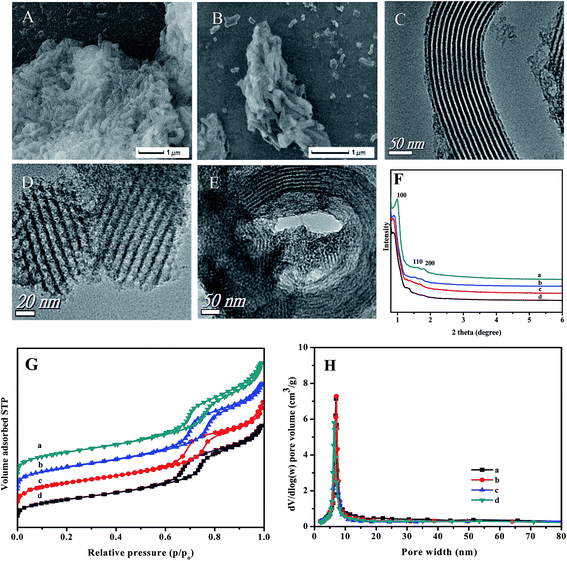
SEM and TEM images of the OMS and C4-IL@OMS prepared via hydrothermal procedure were shown in Fig. 6A–E. Fig. 6A reveals that the as-prepared material OMS possessed rod-like morphologies and relatively uniform size. As shown in Fig. 6B, compared with the parent mesoporous OMS, the rod-like morphologies was maintained after the encapsulation of C4-IL, demonstrating that the encapsulation of the C4-IL had little influence on the formation of the morphologies of catalysts. The similar phenomenon could be found for C8-IL@OMS and C16-IL@OMS (Fig. S1†). TEM images of the rod-like OMS viewed as parallel and perpendicular to the ordered channels were presented in Fig. 6C and D, respectively. The results revealed that the silica rods possessed the one-dimensional (1D) mesoporous channels, long-range order and a hexagonal structure.56 For C4-IL@OMS (Fig. 6E), the ordered mesostructure of the resultant material was still retained, and no aggregation of ILs could be found. The observations obtained above could be further conformed by small-angle XRD (Fig. 6F). For the parent OMS (Fig. 6F(a)), three well-resolved reflections ascribed to (100), (110) and (200) planes for space group P6mm could be found, implying that the two-dimensional (2D) hexagonal mesostructured with long range order. Small-angle XRD patterns of various IL@OMS shows similar reflections compared with the parent OMS, indicating that all IL@OMS possessed an ordered mesoporous structure. It was worth noted that the (100) reflection of the as-synthesized materials IL@OMS shifted to the low angle, and the reflections peaks broadened, which was due to a lattice expansion during the encapsulation of ILs.57 N2 adsorption–desorption isotherms of the neat OMS and the IL@OMS encapsulated with different length alkyl chain POM-based ILs all presented typical type IV curves with a H1 hysteresis loop appeared in 0.6 < p/po <0.9, which suggested that the various catalysts possessed uniform meso-structure. After the encapsulation of ILs into the OMS, the specific surface area of C4-IL@OMS was 630 m2 g−1 (Table 1), slightly smaller than that of the parent OMS (647 m2 g−1), but larger than that of C8-IL@OMS and C16-IL@OMS (590 m2 g−1 and 540 m2 g−1, respectively). Notably, it could be observed that the sorption steps of the as-prepared materials IL@OMS shifted to lower relative pressure as the length of alkyl chain in IL increased, which was ascribed to the pore volume packed with ILs.58 In addition, the various hybrid materials presented narrow pore size distributions around 7 nm obtained from Barrett–Joyner–Halenda (BJH) (Fig. 6H).
| Entry | Sample | SBET (m2 g−1) | Sulfur removal (%) | |
|---|---|---|---|---|
| Catal.a | Catal. + H2O2b | |||
| a Reaction conditions: m(catalyst) = 0.01 g, T = 60 °C, t = 60 min.b Reaction conditions: m(catalyst) = 0.01 g, T = 60 °C, t = 60 min, n(H2O2)/n(S) = 3. | ||||
| 1 | OMS | 647 | 6.1 | 12.2 |
| 2 | C4-IL@OMS | 630 | 4.0 | 99.5 |
| 3 | C8-IL@OMS | 590 | 6.3 | 76.7 |
| 4 | C16-IL@OMS | 540 | 9.6 | 30.5 |
3.2. Catalytic performance
| Entry | POM-based catalyst | O/Sa | t (min) | S-removal (%) | Ref. |
|---|---|---|---|---|---|
| a O/S molar ratio.b m(catalyst) = 0.01 g. | |||||
| 1 | HPMo/HKUST-1 | 6 | 120 | 99 | 60 |
| 2 | PTA/MSN/AEM | 6 | 1320 | 100 | 10 |
| 3 | HPW/NH2-MCM-41 | 8 | 180 | 100 | 61 |
| 4 | [C16H33(CH3)2NOH]3{PO4[WO(O2)2]4}/silica | 4 | 180 | 100 | 62 |
| 5 | HPW/HMS | 8 | 120 | 98 | 63 |
| 6 | (Bu4N)4H3(PW11O39)/MCM-41 | 4 | 60 | 99 | 64 |
| 7 | LaW10/NH3+/SiO2 | 5 | 35 | 99 | 65 |
| 8 | TBA3PW12O40/MIL-101 | 10 | 60 | 100 | 66 |
| 9 | HPW/mpg-C3N4 | 8 | 150 | 100 | 67 |
| 10 | H3PW12O40/MIL-101-(Cr)–NH2 | 4 | 60 | 100 | 68 |
| 11 | PTA/MIL-101 | 50 | 180 | 91 | 69 |
| 12 | LaW10/IL-SiO2 | 5 | 40 | 100 | 11 |
| 13 | [(C6H13)3PC14H29]2W6O19 | 4 | 80 | 99.3 | 9 |
| 14b | C4-IL | 3 | 60 | 12.7 | This work |
| 15b | C4-IL + acetonitrile | 3 | 60 | 31.6 | This work |
| 16b | C4-IL@OMS | 3 | 60 | 99.5 | This work |
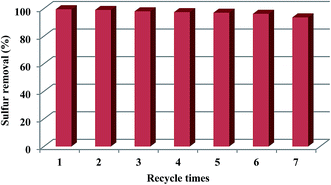 | ||
| Fig. 9 Recycling tests of C4-IL@OMS for the catalytic oxidation of DBT. Reaction conditions: m(C4-IL@OMS) = 0.01 g, O/S = 3, T = 60 °C, t = 60 min. | ||
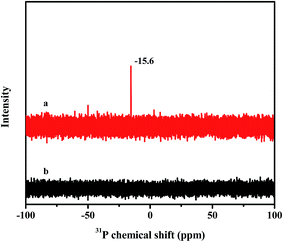
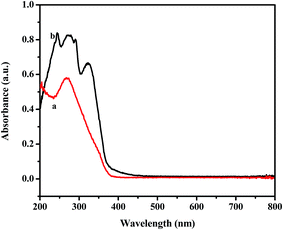
The stability of as-synthesized material C4-IL@OMS in the desulfurization was also performed by 31P NMR and UV-vis spectra. In Fig. 10, a main peak around −15.6 ppm could be observed for the parent C4-IL dissolved in DMSO (Fig. 10a), which was attributed to 31P chemical shift of Keggin structure in the fresh C4-IL.51,73 However, no obvious 31P signal could be observed in the model phase after reaction (Fig. 10b), demonstrating that no leaching of C4-IL into the reaction solution. On the other hand, UV-vis spectra of fresh catalyst and used catalyst were shown in Fig. 11. Besides the absorbance peak concerning the charge transfer from O2− to W6+ for the IL in hybrid material (Fig. 11a), the emerging peaks responsible for the oxidant products of DBT were observed, which also indicated the good stability of the hybrid materials.
4. Conclusions
In summary, a novel catalyst C4-IL@OMS with high specific surface area and ordered mesoporous channels was successfully fabricated by one-pot hydrothermal method and employed in the desulfurization of organosulfurs. The as-synthesized material characterized indicated that the POM-based IL was successfully encapsulated with a highly uniform dispersion in the silica architecture. Additionally, the hybrid material presented excellent activity toward various sulfur-containing compounds, which could be assigned to the hexagonal mesoporous silica with large surface area, enough exposed active species to interact with sulfides, and the chemical property in pore channels. Moreover, the hybrid catalyst still shows high activity after recycling for seven times. 31P NMR and UV-vis spectra after reaction demonstrated that the as-prepared material C4-IL@OMS was highly stable during the reaction. Considering of the advantages for the novel catalyst, it would be further applied in other reactions.Acknowledgements
This work was financially supported by the National Nature Science Foundation of China (No. 21406092, 21376111, 21376109), The Natural Science Foundation of Jiangsu Province (No. BK20131207), Advanced Talents of Jiangsu University (No. 13JDG080), Postdoctoral Foundation of China and Jiangsu Province (No. 2014M551516, 1301001A), and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).References
- M. Zhou, W. Meng, Y. Li, Q. Wang, X. Li and S. Zang, Energy Fuels, 2014, 28, 516–521 CrossRef CAS.
- Y. Liu, S. Liu, S. Liu, D. Liang, S. Li, Q. Tang, X. Wang, J. Miao, Z. Shi and Z. Zheng, ChemCatChem, 2013, 5, 3086–3091 CrossRef CAS PubMed.
- R. Martínez-Palou and R. Luque, Energy Environ. Sci., 2014, 7, 2414–2447 Search PubMed.
- X. Ma, K. Sakanishi and I. Mochida, Ind. Eng. Chem. Res., 1996, 35, 2487–2494 CrossRef CAS.
- R. Javadli and A. de Klerk, Energy Fuels, 2012, 26, 594–602 CrossRef CAS.
- J. Xiong, W. Zhu, H. Li, L. Yang, Y. Chao, P. Wu, S. Xun, W. Jiang, M. Zhang and H. Li, J. Mater. Chem. A, 2015, 3, 12738–12747 CAS.
- N. A. Khan, Z. Hasan and S. H. Jhung, Chem.–Eur. J., 2014, 20, 376–380 CrossRef CAS PubMed.
- J. Xiao, X. Wang, M. Fujii, Q. Yang and C. Song, AIChE J., 2013, 59, 1441–1445 CrossRef CAS PubMed.
- W. Zhu, B. Dai, P. Wu, Y. Chao, J. Xiong, S. Xun, H. Li and H. Li, ACS Sustainable Chem. Eng., 2015, 3, 186–194 CrossRef CAS.
- X. Cui, D. Yao, H. Li, J. Yang and D. Hu, J. Hazard. Mater., 2012, 205, 17–23 CrossRef PubMed.
- Y. Chen and Y. F. Song, ChemPlusChem, 2014, 79, 304–309 CrossRef CAS PubMed.
- H. Lü, P. Li, C. Deng, W. Ren, S. Wang, P. Liu and H. Zhang, Chem. Commun., 2015, 51, 10703–10706 RSC.
- E. Rafiee and N. Nobakht, J. Mol. Catal. A: Chem., 2015, 398, 17–25 CrossRef CAS PubMed.
- A. S. Ogunlaja, W. Chidawanyika, E. Antunes, M. A. Fernandes, T. Nyokong, N. Torto and Z. R. Tshentu, Dalton Trans., 2012, 41, 13908–13918 RSC.
- Y. J. Tian, Y. Yao, Y. H. Zhi, L. J. Yan and S. X. Lut, Energy Fuels, 2015, 29, 618–625 CAS.
- C. Li, D. Li, S. Zou, Z. Li, J. Yin, A. Wang, Y. Cui, Z. Yao and Q. Zhao, Green Chem., 2013, 15, 2793–2799 RSC.
- W. Zhu, C. Wang, H. Li, P. Wu, S. Xun, W. Jiang, Z. Chen, Z. Zhao and H. Li, Green Chem., 2015, 17, 2464–2472 RSC.
- F. Lin, D. Wang, Z. Jiang, Y. Ma, J. Li, R. Li and C. Li, Energy Environ. Sci., 2012, 5, 6400–6406 CAS.
- F. T. Li, Y. Liu, Z. M. Sun, Y. Zhao, R. H. Liu, L. J. Chen and D. S. Zhao, Catal. Sci. Technol., 2012, 2, 1455–1462 CAS.
- A. Nisar and X. Wang, Dalton Trans., 2012, 41, 9832–9845 RSC.
- Y. Leng, J. Zhao, P. Jiang and J. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 5947–5954 CAS.
- A. X. Yan, S. Yao, Y. G. Li, Z. M. Zhang, Y. Lu, W. L. Chen and E. B. Wang, Chem.–Eur. J., 2014, 20, 6927–6933 CrossRef CAS PubMed.
- W. Qi, W. Liu, S. Liu, B. Zhang, X. Gu, X. Guo and D. Su, ChemCatChem, 2014, 6, 2613–2620 CrossRef CAS PubMed.
- Z. Ma, J. Yu and S. Dai, Adv. Mater., 2010, 22, 261–285 CrossRef CAS PubMed.
- Y. Leng, J. Wang, D. Zhu, X. Ren, H. Ge and L. Shen, Angew. Chem., Int. Ed., 2009, 48, 168–171 CrossRef CAS PubMed.
- Y. Ma, S. Qing, L. Wang, N. Islam, S. Guan, Z. Gao, X. Mamat, H. Li, W. Eli and T. Wang, RSC Adv., 2015, 5, 47377–47383 RSC.
- W. Jiang, W. Zhu, Y. Chang, Y. Chao, S. Yin, H. Liu, F. Zhu and H. Li, Chem. Eng. J., 2014, 250, 48–54 CrossRef CAS PubMed.
- H. Lu, S. Wang, C. Deng, W. Ren and B. Guo, J. Hazard. Mater., 2014, 279, 220–225 CrossRef CAS PubMed.
- N. Azgomi and M. Mokhtary, J. Mol. Catal. A: Chem., 2015, 398, 58–64 CrossRef CAS PubMed.
- K. B. Sidhpuria, A. L. Daniel-da-Silva, T. Trindade and J. A. P. Coutinho, Green Chem., 2011, 13, 340–349 RSC.
- P. Sharma and M. Gupta, Green Chem., 2015, 17, 1100–1106 RSC.
- J. D. Patil, S. A. Patil and D. M. Pore, RSC Adv., 2015, 5, 21396–21404 RSC.
- H. Wan, C. Chen, Z. Wu, Y. Que, Y. Feng, W. Wang, L. Wang, G. Guan and X. Liu, ChemCatChem, 2015, 7, 441–449 CrossRef CAS PubMed.
- C. Liu, G. Zhang, C. Zhao, X. Li, M. Li and H. Na, Chem. Commun., 2014, 50, 14121–14124 RSC.
- Y. Ding, B. Zhang, N. Gupta and D. S. Su, Green Chem., 2015, 17, 1107–1112 RSC.
- X. Fang, Z. Liu, M. F. Hsieh, M. Chen, P. Liu, C. Chen and N. Zheng, ACS Nano, 2012, 6, 4434–4444 CrossRef CAS PubMed.
- Q. He, Y. Gao, L. Zhang, W. Bu, H. Chen, Y. Li and J. Shi, J. Mater. Chem., 2011, 21, 15190–15192 RSC.
- J. M. Palomino, D. T. Tran, J. L. Hauser, H. Dong and S. R. J. Oliver, J. Mater. Chem. A, 2014, 2, 14890–14895 CAS.
- J. Zhang, A. Wang, X. Li and X. Ma, J. Catal., 2011, 279, 269–275 CrossRef CAS PubMed.
- P. Virtanen, J. P. Mikkola, E. Toukoniitty, H. Karhu, K. Kordas, K. Eränen, J. Wärnå and T. Salmi, Catal. Today, 2009, 147, 144–148 CrossRef PubMed.
- A. Bordoloi, S. Sahoo, F. Lefebvre and S. Halligudi, J. Catal., 2008, 259, 232–239 CrossRef CAS PubMed.
- S. M. Sadeghzadeh, Green Chem., 2015, 17, 3059–3066 RSC.
- A. Eguizabal, J. Lemus, M. Urbiztondo, A. M. Moschovi, S. Ntais, J. Soler and M. P. Pina, J. Power Sources, 2011, 196, 4314–4323 CrossRef CAS PubMed.
- W. Zhu, W. Huang, H. Li, M. Zhang, W. Jiang, G. Chen and C. Han, Fuel Process. Technol., 2011, 92, 1842–1848 CrossRef CAS PubMed.
- K. Li, J. Hu, W. Li, F. Ma, L. Xu and Y. Guo, J. Mater. Chem., 2009, 19, 8628–8638 RSC.
- M. Zhang, W. S. Zhu, S. H. Xun, H. M. Li, Q. Q. Gu, Z. Zhao and Q. Wang, Chem. Eng. J., 2013, 220, 328–336 CrossRef CAS PubMed.
- Z. Long, Y. Zhou, W. Ge, G. Chen, J. Xie, Q. Wang and J. Wang, ChemPlusChem, 2014, 79, 1590–1596 CrossRef CAS PubMed.
- H. Zhao, L. Zeng, Y. Li, C. Liu, B. Hou, D. Wu, N. Feng, A. Zheng, X. Xie, S. Su and N. Yu, Microporous Mesoporous Mater., 2013, 172, 67–76 CrossRef CAS PubMed.
- L. S. Nogueira, S. Ribeiro, C. M. Granadeiro, E. Pereira, G. Feio, L. Cunha-Silva and S. S. Balula, Dalton Trans., 2014, 43, 9518–9528 RSC.
- A. Patel and S. Singh, Microporous Mesoporous Mater., 2014, 195, 240–249 CrossRef CAS PubMed.
- A. Mouret, L. Leclercq, A. Mühlbauer and V. Nardello-Rataj, Green Chem., 2014, 16, 269–278 RSC.
- S. Xun, W. Zhu, D. Zheng, L. Zhang, H. Liu, S. Yin, M. Zhang and H. Li, Fuel, 2014, 136, 358–365 CrossRef CAS PubMed.
- M. Zhang, W. S. Zhu, H. M. Li, S. Xun, W. J. Ding, J. J. Liu, Z. Zhao and Q. Wang, Chem. Eng. J., 2014, 243, 386–393 CrossRef CAS PubMed.
- X. Luo and C. Yang, Phys. Chem. Chem. Phys., 2011, 13, 7892–7902 RSC.
- X. Wang, W. Chen and Y. F. Song, Eur. J. Inorg. Chem., 2014, 2779–2786 CrossRef PubMed.
- D. Elhamifar, M. Nasr-Esfahani, B. Karimi, R. Moshkelgosha and A. Shábani, ChemCatChem, 2014, 6, 2593–2599 CrossRef CAS PubMed.
- B. Li, W. Ma, J. Liu, S. Zuo and X. Li, J. Colloid Interface Sci., 2011, 362, 42–49 CrossRef CAS PubMed.
- D. Gu, W. Li, F. Wang, H. Bongard, B. Spliethoff, W. Schmidt, C. Weidenthaler, Y. Xia, D. Zhao and F. Schuth, Angew. Chem., Int. Ed., 2015, 54, 7060–7064 CrossRef CAS PubMed.
- J. Xu, S. Zhao, Y. Ji and Y. F. Song, Chem.–Eur. J., 2013, 19, 708–714 Search PubMed.
- E. Rafiee and N. Nobakht, J. Mol. Catal. A: Chem., 2015, 398, 17–25 CrossRef CAS PubMed.
- G. Luo, L. Kang, M. Zhu and B. Dai, Fuel Process. Technol., 2014, 118, 20–27 CrossRef CAS PubMed.
- H. Zheng, Z. Sun, X. Chen, Q. Zhao, X. Wang and Z. Jiang, Appl. Catal., A, 2013, 467, 26–32 CrossRef CAS PubMed.
- B. Li, W. Ma, J. Liu, C. Han, S. Zuo and X. Li, Catal. Commun., 2011, 13, 101–105 CrossRef CAS PubMed.
- Z. E. A. Abdalla and B. Li, Chem. Eng. J., 2012, 200, 113–121 CrossRef PubMed.
- Y. Chen, S. Zhao and Y. F. Song, Appl. Catal., A, 2013, 466, 307–314 CrossRef CAS PubMed.
- S. Ribeiro, A. D. S. Barbosa, A. C. Gomes, M. Pillinger, I. S. Goncalves, L. Cunha-Silva and S. S. Balula, Fuel Process. Technol., 2013, 116, 350–357 CrossRef CAS PubMed.
- Y. Zhu, M. Zhu, L. Kang, F. Yu and B. Dai, Ind. Eng. Chem. Res., 2015, 54, 2040–2047 CrossRef CAS.
- X. S. Wang, Y. B. Huang, Z. J. Lin and R. Cao, Dalton Trans., 2014, 43, 11950–11958 RSC.
- X. Hu, Y. Lu, F. Dai, C. Liu and Y. Liu, Microporous Mesoporous Mater., 2013, 170, 36–44 CrossRef CAS PubMed.
- Y. Jia, G. Li, G. Ning and C. Jin, Catal. Today, 2009, 140, 192–196 CrossRef CAS PubMed.
- K. S. Cho and Y. K. Lee, Appl. Catal., B, 2014, 147, 35–42 CrossRef CAS PubMed.
- S. Otsuki, T. Nonaka, N. Takashima, W. H. Qian, A. Ishihara, T. Imai and T. Kabe, Energy Fuels, 2000, 14, 1232–1239 CrossRef CAS.
- L. H. Wee, F. Bonino, C. Lamberti, S. Bordiga and J. A. Martens, Green Chem., 2014, 16, 1351–1357 RSC.
- Y. Leng, J. Wang, D. Zhu, M. Zhang, P. Zhao, Z. Long and J. Huang, Green Chem., 2011, 13, 1636–1639 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra13787j |
| This journal is © The Royal Society of Chemistry 2015 |