Bis-imidazolium and benzimidazolium based gemini-type ionic liquids structure: synthesis and antibacterial evaluation†
Nassir N. Al-Mohammed*,
Yatimah Alias* and
Zanariah Abdullah
Chemistry Department, Faculty of Science, University of Malaya, Lembah Pantai, Kuala Lumpur 50603, Malaysia. E-mail: nassir@siswa.um.edu.my; nassirnn@gmail.com; yatimah70@um.edu.my; Fax: +60-3-79677188
First published on 5th October 2015
Abstract
Based on bis-imidazolium and benzimidazolium, new sets of geminal dicationic ionic liquids containing a sulphonamide moiety were successfully synthesized with good yields. Their structures were confirmed by 1H-NMR, 13C-NMR, FT-IR, and mass spectroscopy. Selected physicochemical properties of these ILs including thermal stability by TGA and miscibility in some common organic solvents and water were also determined. Most of the prepared dicationic ILs displayed significant levels of antibacterial activities against ten selected bacterial strains of Gram-positive and Gram-negative using a micro-broth dilution assay.
1. Introduction
Ionic liquids (ILs) are salts typically consisting of large organic cations containing various substituents1,2 associated with organic or inorganic anions.3,4 They exhibit several unique chemical and physical properties, such as extremely low vapour pressure, low melting point, non-flammability, wide electrochemical window, excellent solvation and high thermal stability. Further, through the modification of the cation and anion, ILs can be tuned to be miscible with either low polarity organic solvents including hexanes, toluene, ether, supercritical CO2 or high polarity solvents such as water and ethanol.5 The interest in ILs as ‘greener’ solvents has dramatically expanded to include a wide unexpected range of applications that have been reported in synthesis and biotechnology.ILs with a molecular structure of gemini surfactants (geminal ILs) are a new class of amphiphilic molecules containing two head groups (two identical or dissimilar cationic moieties) and two aliphatic chains, linked by a rigid or flexible spacer. Compared to traditional ILs, geminal dicationic liquids have shown superior physical properties6–9 in thermal stability, solubility in aqueous media, high density, interface property, lower critical micelle concentration (CMC), and unusual rheological properties. Accordingly, they have multiple promising applications in life science, petro-chemistry, medicine, etc. Further, dicationic ILs as multifunctional ions have an exclusive approach to “tune” or alter their physicochemical properties to a greater range than more traditional monocationic ILs. The “tenability” or structural variations include the effect of the cationic part symmetry (i.e., identical or not), the length and type of both spacer and the side chains, as well as the type of counter-anions.
Recently, several ammonium-based dicationic phosphate salt liquids have been prepared and characterized.10–13 In the meanwhile, some dicationic ILs based on imidazolium pyridinium and ammonium with polyether linker,14 have been synthesized by Ohno and co-workers.15 Additionally, two studies by Anderson et al.,7 and Payagala et al.,9 deal with the synthesis and physicochemical property manipulation of symmetrical and unsymmetrical geminal dicationic ILs, respectively. They have characterized several properties including thermal stability, surface tension, density, miscibility with a polar and nonpolar solvent, and shear viscosity of imidazolium and pyrrolidinium cation based ILs. Thermal stabilities of the symmetrical and unsymmetrical dicationic ILs are higher than their corresponding conventional monocationic ILs. Precisely, imidazolium and pyridinium geminal dicationic ILs have shown an increased thermal stability, with onset temperatures of thermal decomposition (Tonset) about 150 °C above the decomposition temperature of the monocationic ILs.7,9 Thermogravimetric analysis (TGA) at elevated temperatures is used to evaluate thermal stability of many dicationic ILs. This method of short-term stability, called the ramped temperature analysis method (also called step-tangent or dynamic analysis) with most commonly uses heating rates of 10 °C min−1 and 20 °C min−1.16,17 Multiple factors including the great charge and intermolecular interactions, density, molecular weight and shear viscosity associated with a small free volume, were used to explain the observed high thermal stability of dicationic ILs.18,19
From the IL structural point of view, poly-functionalized heterocyclic compounds containing imidazole and its derivatives are acquiring more importance due to their biological activity. Most ILs contain heterocyclic derivatives as cations, e.g. imidazolium, benzimidazolium, pyridinium, pyrrolium, pyrrolidinium and ILs with bridged structures. Drug designs based on the high therapeutic properties of the imidazole and benzimidazole are considered as an advantage towards the synthesis of a number of novel clinical agents against various types of diseases. Moreover, extensive biochemical and pharmacological studies have confirmed imidazole and benzimidazole as effective compounds in treating various strains of microorganisms.20–24 Their antibacterial and antifungal effects are attributed to cationic interactions with negatively charged parts of bacterial membranes.25 Furthermore, the benzenesulfonamide moiety is well known for its several pharmacological activities, individually or when incorporated with other bioactive moieties within the same molecule.26–28 Typically, sulfonamide compounds are widely studied due to their chemotherapeutic and interesting properties related to antibacterial,29,30 anti-inflammatory,31,32 analgesic agents,33 antifungal34,35 and antiviral activity.36,37 Molecular modeling and the Quantitative Structure–Activity Relationship (QSAR) method38–41 have been used to confirm their antibacterial activity for many applications in bio-inorganic and metal-based drug chemistry.42–45 The enhancement of antibacterial activity of di-imidazole and di-benzimidazole compounds incorporated with a sulfonamide moiety has been reported by authors of previous work.46 However, much of the research dealt with geminal di-cationic IL synthesis when it comes to their design,6,7,9,18,47–52 while limited studies have considered dicationic ILs with a highly rigid spacer.53,54 The current work concentrates on the synthesis of novel geminal bis-imidazolium and benzimidazolium ILs consisting of two substituent symmetric head groups (two identical cations), linked by a high rigidity spacer containing a benzenesulfonamide moiety in high yield and purity. To explore the structure–activity relationship (SAR) of this novel dicationic IL series which contain multi bioactive moieties, an in vitro antibacterial evaluation of halogen ILs against standard strains of six Gram positive and four Gram negative bacteria, are investigated. Further, the thermal stability and miscibility of the prepared ILs are indicated as well. The presence of an incorporated benzenesulfonamide moiety as well as the active side substituents into di-imidazolium and benzimidazolium cations enhanced both antibacterial activity and miscibility for the synthesized ILs. The effects of anions on antibacterial activity and thermal stability are beyond the scope of this study.
2. Results and discussion
2.1. Synthesis
Compounds N,N-bis[(imidazol-1-yl)ethyl]-4-methylbenzenesulfonamide (3) and N,N-bis[(benzimidazol-1-yl)ethyl]-4-methylbenzenesulfonamide (4) were previously synthesized in sufficient purity46 and currently used as precursors to produce bis-imidazolium and bis-benzimidazolium dicationic ILs, respectively. The highly reactive halides; allyl bromide, propargyl bromide, chloroacetonitrile, 2-bromoethanol, ethyl bromoacetate and tert-butyl bromoacetate were used for the alkylation reactions, thus, the high yields were not surprising due to the easy substitution reaction with both imidazole and benzimidazole rings, Scheme 1.In the current work, all the synthesized halogen (chloride or bromide) ILs are semi-solid to syrup or viscous liquid at room temperature which has been considered as a criteria to determine their classification as ILs.55,56 Generally, ILs tend to be liquid at room temperature, which is attributed to the high conformational degrees of freedom. Moreover, the NTf2 counter ion confers lower viscosity and decreased melting point compared with halogen precursors. Metathesis of the halogen anion to NTf2− produced clear liquids at room temperature and clean samples were isolated after a simple workup. The process of counter-ion exchange involved stirring an aqueous solution of the halogen IL with LiNTf2 for a few hours. A good yield of the hydrophobic IL phase was then separated by extraction with ethyl acetate to produce pure and clear liquid samples of the IL after organic layer evaporation under reduced pressure. The purity of the NTf2-ILs was confirmed by 13C and 9F-NMR. Table 1 summarizes the synthetic details of the prepared ILs.
| IL | Cation | Incorporated side groups | Counter ions | Statusc | M. wt | Yield (%) |
|---|---|---|---|---|---|---|
| a Imidazolium.b Benzimidazolium.c At room temperature. | ||||||
| 5a | Ima | –CH2CHCH2 | Br− | Syrup | 601.40 | 95 |
| 5b | Ima | –CH2CCH | Br− | Syrup | 597.37 | 98 |
| 5c | Ima | –CH2CN | Cl− | Liquid | 510.44 | 98 |
| 5d | Ima | –CH2CH2OH | Br− | Liquid | 609.37 | 99 |
| 5e | Ima | –CH2CO2C2H5 | Br− | Semi-solid | 693.45 | 96 |
| 5f | Ima | –CH2CO2C(CH3)3 | Br− | Semi-solid | 749.55 | 95 |
| 6a | BImb | –CH2CHCH2 | Br− | Liquid | 701.51 | 95 |
| 6b | BImb | –CH2CCH | Br− | Syrup | 697.48 | 98 |
| 6c | BImb | –CH2CN | Cl− | Syrup | 610.56 | 94 |
| 6d | BImb | –CH2CH2OH | Br− | Liquid | 709.49 | 98 |
| 6e | BImb | –CH2CO2C2H5 | Br | Syrup | 793.57 | 98 |
| 6f | BImb | –CH2CO2C(CH3)3 | Br− | Syrup | 849.67 | 98 |
| 7d | Ima | –CH2CH2OH | NTf2− | Liquid | 1009.35 | 83 |
| 7e | Ima | –CH2CO2C2H5 | NTf2− | Liquid | 1093.93 | 96 |
| 7f | Ima | –CH2CO2C(CH3)3 | NTf2− | Liquid | 1150.03 | 95 |
| 8a | BImb | –CH2CHCH2 | NTf2− | Liquid | 1101.99 | 90 |
| 8d | BImb | –CH2CH2OH | NTf2− | Liquid | 1109.98 | 87 |
| 8f | BImb | –CH2CO2C(CH3)3 | NTf2− | Liquid | 1250.16 | 97 |
The spectral data (IR, 1H, 13C, 19F-NMR and mass) are in good agreement with current proposed structures of the newly synthesized ILs. FT-IR spectra for all synthesized ILs (i.e. 5a–f, 6a–f, 7d–f and 8a–f) showed absorption bands at 1329–1364 cm−1 and 1151–1156 cm−1 which were assigned to the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group. These bis-imidazolium and benzimidazolium ILs showed stretching absorption bands at 3142–3027 cm−1, 2990–2850 cm−1, 1644–1590 cm−1, and 1566–1443 cm−1 attributed to (C–H)Aromatic, (C–H)Aliphatic, (C
O group. These bis-imidazolium and benzimidazolium ILs showed stretching absorption bands at 3142–3027 cm−1, 2990–2850 cm−1, 1644–1590 cm−1, and 1566–1443 cm−1 attributed to (C–H)Aromatic, (C–H)Aliphatic, (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), and (C
N), and (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)Aromatic, respectively. The bands at 2125 cm−1 and 2121 cm−1 for compounds 5b and 6b were assigned to (C
C)Aromatic, respectively. The bands at 2125 cm−1 and 2121 cm−1 for compounds 5b and 6b were assigned to (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) in propargyl substituents, while 5c and 6c ILs showed characteristic stretching absorption bands at 2238 cm−1 and 2235 cm−1, respectively, which were assigned to (C
C) in propargyl substituents, while 5c and 6c ILs showed characteristic stretching absorption bands at 2238 cm−1 and 2235 cm−1, respectively, which were assigned to (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N). Incorporating ethanol groups into 5d, 6d, 7d, and 8d ILs showed (O–H) bands at 3280–3312 cm−1. The IR spectra of compounds 5e–f, 6e–f 7e–f and 8f showed sharp absorption bands at 1739–1748 cm−1 which were attributed to a carbonyl stretching frequency corresponding to the ester groups. In the 1H-NMR spectra, α-CH2 protons appeared as a singlet (compounds 5c, 5e, 5f, 6c, 6e, 6f, 7e, 7f and 8f), doublet (5a, 5b, 6a, 6b, 8d) and triplet (5d, 6d, 7d and 8d) at δ 5.16–6.13 ppm, δ 4.88–5.58 ppm, and δ 4.19–4.57, respectively. Moreover, singlet peaks appeared in the range of δ 9.72–10.31 ppm corresponding to the isolated C–H of benzimidazolium rings while these protons showed broad triplet–singlet peaks in all imidazolium ILs. The chemical shifts of the imidazole and benzimidazole ring protons in both imidazolium and benzimidazolium ILs are consistently downfield in comparison to the analogous chemical shifts of the core di-imidazole and di-benzimidazole compounds.46 These observations are in accord with the presence of positive charges in both IL types, where the higher shifts were recorded with acetonitrile as the active side groups; compounds 5c and 6c. The allylic-CH in compounds 5a, 6a and 8a showed characteristic multiplet peaks in the range of δ 5.98–6.15 ppm, while the allylic-CH2 showed four individual doublet peaks with different J constants: 0.98, 1.22 and 1.36 Hz. Further, compounds 5b and 6b presented triplet peaks at δ 3.88 and 3.91 ppm, which were attributed to propargyl-CH with J constant values of 2.72 and 2.27, respectively. The peak of O–H protons for compounds 5d, 6d, 7d and 8d appeared as a broad singlet at δ 5.15–5.22 ppm integrating for two protons. In general, imidazolium ILs showed up-field resonances when compared to benzimidazolium ILs with both halogen and NFf2 anions.
N). Incorporating ethanol groups into 5d, 6d, 7d, and 8d ILs showed (O–H) bands at 3280–3312 cm−1. The IR spectra of compounds 5e–f, 6e–f 7e–f and 8f showed sharp absorption bands at 1739–1748 cm−1 which were attributed to a carbonyl stretching frequency corresponding to the ester groups. In the 1H-NMR spectra, α-CH2 protons appeared as a singlet (compounds 5c, 5e, 5f, 6c, 6e, 6f, 7e, 7f and 8f), doublet (5a, 5b, 6a, 6b, 8d) and triplet (5d, 6d, 7d and 8d) at δ 5.16–6.13 ppm, δ 4.88–5.58 ppm, and δ 4.19–4.57, respectively. Moreover, singlet peaks appeared in the range of δ 9.72–10.31 ppm corresponding to the isolated C–H of benzimidazolium rings while these protons showed broad triplet–singlet peaks in all imidazolium ILs. The chemical shifts of the imidazole and benzimidazole ring protons in both imidazolium and benzimidazolium ILs are consistently downfield in comparison to the analogous chemical shifts of the core di-imidazole and di-benzimidazole compounds.46 These observations are in accord with the presence of positive charges in both IL types, where the higher shifts were recorded with acetonitrile as the active side groups; compounds 5c and 6c. The allylic-CH in compounds 5a, 6a and 8a showed characteristic multiplet peaks in the range of δ 5.98–6.15 ppm, while the allylic-CH2 showed four individual doublet peaks with different J constants: 0.98, 1.22 and 1.36 Hz. Further, compounds 5b and 6b presented triplet peaks at δ 3.88 and 3.91 ppm, which were attributed to propargyl-CH with J constant values of 2.72 and 2.27, respectively. The peak of O–H protons for compounds 5d, 6d, 7d and 8d appeared as a broad singlet at δ 5.15–5.22 ppm integrating for two protons. In general, imidazolium ILs showed up-field resonances when compared to benzimidazolium ILs with both halogen and NFf2 anions.
13C-NMR was used to assign the carbon skeleton of the synthesized geminal dicationic imidazolium and benzimidazolium ILs. The PENDANT experiment (polarization enhancement nurtured during attached nucleus testing) was applied to differentiate between the methylene (CH2) and methine (CH) carbon signals based on different H-content of carbon atoms that have environment similarity.57 In the PENDANT spectra methyl (CH3) and methine (CH) carbons appear as positive signals, while methylene (CH2) and quaternary carbon (C) show negative signals. Fig. 1 shows the 13C-NMR PENDANT spectrum of IL 6a.
In the 13C-NMR spectra of 5a, 6a, and 8a ILs, the signals around δ 130 ppm and 120 ppm were assigned to allylic CH and CH2, respectively, while the propargyl active side groups in both 5b and 6b ILs showed characteristic peaks at δ 79 ppm for –C– and δ 75 ppm for CH. Additional signals were observed at 114 ppm and 113 ppm, which were assigned to the carbon atom of CN for compounds 5c and 6c, respectively. The peaks recorded at δ 165–167 ppm, were attributed to carbon atom of carbonyl groups in compounds 5e, 5f, 6e, 6f, 7e, 7f and 8f. Ethanolic carbon atoms in 5d, 6d, 7d and 8d ILs were determined at 59 ppm. Carbon atoms C–F in 7d–f, 8a, 8d and 8f ILs, showed quartet peaks with 320 Hz constant J values within the range of δ 126–114 ppm. The characteristic chemical shifts for F/CF3 were also detected in the 19F-NMR spectra at −80 ppm. With the high resolution mass spectra, the identity of the bis-imidazolium and benzimidazolium ILs was confirmed as a [M+2 − H]–2X−; (M = cation and X = anion) in both kinds of IL anions.
2.2. Solubility
Solubility behaviour of all the synthesized geminal dicationic ILs in water and common organic solvents was evaluated at room temperature. These solvents have a wide range of polarity from highly polar, water or alcohols, gradually to weakly or non-polar solvents like toluene or hexane, respectively. The observations obtained from solubility tests are summarized in Table 2.| IL | Water | Ethanol | Acetone | Ethyl acetate | Tetrahydrofuran | Chloroform | Toluene | Hexane |
|---|---|---|---|---|---|---|---|---|
| a (+) miscible – a drop of the compound dissolves in a few drops (1–5) of solvent, (±) moderately miscible – dissolves in more than 10 drops of solvent, (−) immiscible – did not dissolve in 1–2 mL of solvent. | ||||||||
| 5a | + | + | + | + | + | + | + | − |
| 5b | + | + | + | + | + | + | + | − |
| 5c | + | + | + | + | + | + | ± | − |
| 5d | + | + | + | + | + | + | ± | − |
| 5e | + | + | + | + | + | + | + | − |
| 5f | + | + | + | + | + | + | + | − |
| 6a | + | + | + | + | + | + | + | − |
| 6b | + | + | + | + | + | + | + | − |
| 6c | + | + | + | + | + | + | + | − |
| 6d | + | + | + | + | + | + | + | − |
| 6e | + | + | + | + | + | + | + | − |
| 6f | + | + | + | + | + | + | + | − |
| 7d | − | ± | + | + | + | + | ± | − |
| 7e | − | − | + | + | + | + | + | − |
| 7f | − | − | + | + | + | + | + | − |
| 8a | − | − | + | + | + | + | + | − |
| 8d | − | ± | + | + | + | + | + | − |
| 8f | − | − | + | + | + | + | + | − |
The IL was considered miscible (if a drop of the IL dissolves in a few drops (1–5) of the solvent), partially miscible (if it dissolves in more than 10 drops of the solvent), or immiscible (if it did not dissolve in 2 mL of the solvent)58 which are termed (+), (±) and (−), respectively. It can be observed that all halogen bis-imidazolium and benzimidazolium ILs are miscible with water while reverse miscibility was noticed for the ILs incorporating the NTf2− anion. All ILs studied for both kinds of anions are totally miscible with acetone, ethyl acetate, tetrahydrofuran and chloroform, while they are shown to be immiscible with hexane. Generally, the introduction of hydroxyl in the side active groups considerably modified the solubility behaviour of ILs 7d and 8d with ethanol,59 while no significant influence have been noticed for the rest of the ILs when other functional groups changed or in the introduction of a benzene ring into the imidazolium ILs.
The solubility behaviour of the geminal dicationic ILs in water and all common organic solvents was significantly similar to mono-cationic ILs,60–62 whereas the halogen and NTf2− dicationic ILs were noticed as miscible and immiscible with water, respectively. Obviously, the presence of a hydrophobic anion (NTf2−) exceeds the coordinating nature of the bromide (or chloride) anion to produce immiscible ILs with water. Thus, the individual cations and anions can be tuneable to produce ILs with the desired properties and characteristics.
2.3. Antibacterial activities
A microbroth dilution bioassay was used to evaluate the antibacterial activities of the synthesized halogen imidazolium and benzimidazolium geminal dicationic ILs against representative standard strains of Gram-positive and Gram-negative bacteria. Minimum inhibitory concentrations MIC (mg mL−1) of the studied dicationic ILs were determined, and the results are listed in Table 3. The majority of these ILs showed significant antibacterial activity towards most of the selected microorganisms as shown in Fig. 2. According to studies of the structure–activity relationship (SAR), the incorporation of two different pharmacophores within the same molecule would enhance the resulting compounds’ biological activities.37,63,64 Therefore, the presence of an incorporated benzenesulfonamide moiety adjacent to the imidazolium and benzimidazolium core in the ILs, successfully promoted the antibacterial activity of the produced geminal dicationic ILs. Based on antimicrobial activity studies of imidazolium ILs, the halogen anions showed the least toxicity,21,65–68 while IL bioactivity is largely driven by hydrophobicity and active side substitutions (or alkyl chain branching) of the cations.69–73 Due to the similarity of IL structures to detergents, pesticides and antibiotics, the proposed mechanism of ILs toxicity is through membrane disruption where the toxic effect may be related to a common cellular structure or process.21,74 Further, ILs as cationic surfactants may cause disruption in membrane-bound proteins due to their interfacial properties, and the induced polar narcosis effect.75 The current ILs substance attacked the lipid structure of the membrane (lipo-polysaccharide layer), where the sulfonyl group of the sulfonamide moiety interfered with cell metabolism. Thus, compared to the previously prepared core compounds46 (3 and 4), the cationic substitutions of the geminal dicationic ILs, have successfully enhanced the biological activities of both imidazolium and benzimidazolium ILs. Furthermore, imidazolium ILs showed the highest activities which could be attributed to their higher solubility in water (Table 2). The highest antibacterial toxicity was found for ILs with acetonitrile substituents (5c and 6c), while the ILs of tert-butyl-ester (5f and 6f) did not display dramatic acute biological activities at the selected concentration range. Against β-lactam resistant Gram-negative bacterium (Pseudomonas aeruginosa), all IL compounds except 5b showed significant activities in MIC values of 0.1–0.5 mg mL−1 when compared to the antibiotic amoxicillin. Moreover, 5c and 6c ILs showed considerable antibacterial activities of 0.05 mg mL−1 MIC value against Bacillus subtilis, which required a high dose of amoxicillin76 (0.25 mg mL−1). The antibacterial results of the most tested geminal dicationic ILs demonstrated interesting inhibitory values against Staphylococcus epidermidis. The range of MIC values for tested compounds were between 0.05 and 0.5 mg mL−1, for 6c and 5e, respectively, while β-lactam antibiotic amoxicillin displayed no activity against this strain of Gram-positive bacteria. Against Acinetobacter calcoaceticus Gram-negative bacteria, IL 5c exhibited a significant antibacterial inhibitory effect at 0.05 mg mL−1, while both commercial antibiotics amoxicillin and kanamycin exhibited MIC values of 0.15 mg mL−1 and >0.5 mg mL−1, respectively. Moreover, Enterococcus faecalis showed no effect from the antibiotic kanamycin at the concentration range of the current study (0.05–0.5 mg mL−1), while the dicationic IL compounds 5d, 5e, and 6e demonstrated an inhibitory effect with values of 0.25, 0.25 and 0.3 mg mL−1, respectively against this Gram-positive bacterium. Based on the bioactive compound results, different compounds reacted variously against bacteria. For these dicationic ILs, strains of Gram-positive bacteria seem to be more sensitive than Gram negative micro-organisms.| No. | Structure of sample | Bacteria/MICs (mg mL−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gram-negative bacteria | Gram-positive bacteria | ||||||||||
| Escherichia coli | Salmonella typhimurium | Pseudomonas aeruginosa | Acinetobacter calcoaceticus | Streptococcus pyogenes | Staphylococcus aureus | Bacillus subtilis | Rhodococcus ruber | Enterococcus faecalis | Staphylococcus epidermidis | ||
| a MIC: minimum inhibitory concentration, AM: amoxicillin, KA: kanamycin, nd: not detected. | |||||||||||
| 5a |  |
0.25 | 0.40 | 0.30 | 0.30 | 0.10 | 0.25 | 0.35 | 0.40 | >0.50 | 0.30 |
| 5b |  |
0.50 | >0.50 | >0.50 | 0.50 | 0.30 | 0.35 | >0.50 | 0.40 | >0.50 | >0.50 |
| 5c |  |
0.05 | 0.05 | 0.10 | 0.05 | 0.30 | 0.10 | 0.05 | 0.05 | 0.10 | 0.15 |
| 5d |  |
0.15 | 0.20 | 0.15 | 0.25 | 0.25 | 0.20 | 0.25 | 0.15 | 0.25 | 0.20 |
| 5e |  |
0.40 | 0.35 | 0.30 | 0.30 | >0.50 | 0.50 | 0.35 | 0.20 | 0.25 | 0.50 |
| 5f | 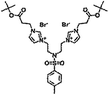 |
0.50 | 0.40 | 0.30 | 0.40 | >0.50 | >0.50 | 0.50 | >0.5 | >0.5 | >0.5 |
| 6a | 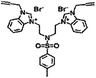 |
0.40 | >0.50 | 0.50 | 0.45 | 0.40 | 0.35 | 0.40 | >0.50 | >0.50 | 0.40 |
| 6b | 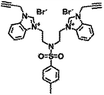 |
>0.50 | >0.50 | 0.50 | >0.50 | 0.45 | 0.50 | >0.50 | >0.50 | >0.50 | >0.50 |
| 6c | 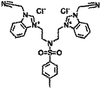 |
0.25 | 0.05 | 0.25 | 0.30 | 0.10 | 0.15 | 0.05 | 0.20 | 0.15 | 0.05 |
| 6d | 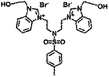 |
0.35 | 0.20 | 0.40 | 0.35 | 0.40 | 0.40 | 0.30 | 0.30 | >0.50 | 0.40 |
| 6e | 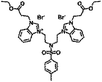 |
0.30 | 0.35 | 0.45 | 0.30 | 0.40 | 0.40 | 0.35 | 0.40 | 0.30 | 0.30 |
| 6f | 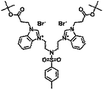 |
>0.5 | >0.5 | 0.4 | >0.5 | 0.5 | >0.5 | >0.5 | >0.5 | >0.5 | >0.5 |
| AM | 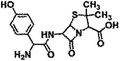 |
<0.05 | <0.05 | nd | 0.15 | 0.05 | <0.05 | 0.25 | <0.05 | <0.05 | nd |
| KA |  |
<0.05 | <0.05 | <0.05 | >0.5 | <0.05 | <0.05 | <0.05 | <0.05 | >0.5 | <0.05 |
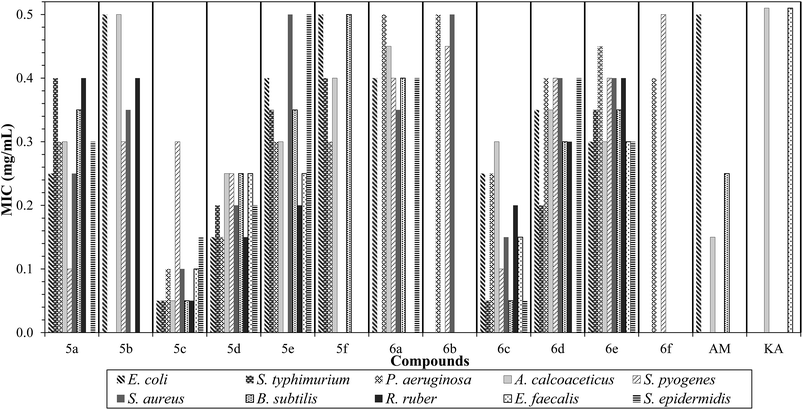 | ||
| Fig. 2 MIC histogram for synthesized ILs (0.05–0.50 mg mL−1 concentration) versus ten strains of bacteria. | ||
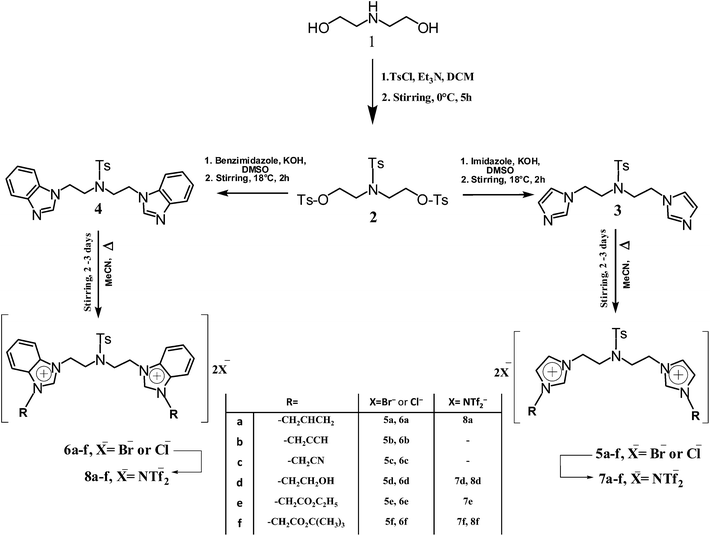
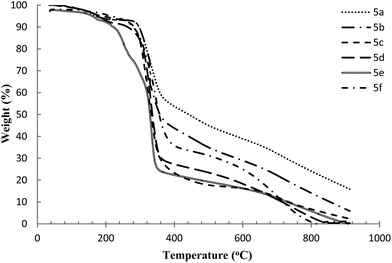
2.4. Thermal stability
The ramped temperature TGA method16,17,77 (at heating rate of 10 °C min−1), was used to measure the decomposition temperatures of the synthesized halogen imidazolium and benzimidazolium geminal dicationic ILs (i.e. 5a–f and 6a–f). The ramped temperature experiment (also called step-tangent or dynamic analysis)7,78 gives rise to a point of thermal degradation, which is termed Tonset the onset point of decomposition and defined as the value of the intercept of two linear functions: the baseline of zero weight loss and the tangent of weight vs. temperature upon decomposition and calculated using thermal analysis software.79 The actual degradation already starts at a lower temperature (Tstart) than the Tonset.80 Typically, the ramped temperature method is also characterized by a temperature of maximum degradation (Tpeak) in between 10 to 100 °C higher than Tonset.16 Moreover T10% or T50%, which reveals the temperature at a weight loss of 10% and 50%, respectively, were reported and several research works have followed a similar technique.81,82 The decomposition temperatures (T10%), (T50%), (Tstart), (Tonset) as well as the differential peak temperature (Tpeak), for all samples are listed in Table 4.| IL | Incorporated side groups | Temperature (°C) corresponding to | ||||
|---|---|---|---|---|---|---|
| Tstarta | T10b | T50c | Tpeakd | Tonsete | ||
| Decomposition temperatures, (°C), corresponding to:a the started decomposition,b at 10% weight loss,c at 50% weight loss,d differential peak,e the onset of decomposition. | ||||||
| 5a | –CH2CHCH2 | 270 | 297 | 299 | 324 | 285 |
| 5b | –CH2CCH | 260 | 288 | 310 | 326 | 285 |
| 5c | –CH2CN | 265 | 285 | 300 | 322 | 294 |
| 5d | –CH2CH2OH | 248 | 262 | 337 | 330 | 289 |
| 5e | –CH2CO2C2H5 | 203 | 235 | 307 | 333 | 233 |
| 5f | CH2CO2C(CH3)3 | 229 | 279 | 300 | 347 | 274 |
| 6a | –CH2CHCH2 | 250 | 277 | 303 | 338 | 274 |
| 6b | –CH2CCH | 265 | 287 | 295 | 324 | 286 |
| 6c | –CH2CN | 213 | 250 | 265 | 333 | 252 |
| 6d | –CH2CH2OH | 255 | 282 | 300 | 322 | 281 |
| 6e | –CH2CO2C2H5 | 200 | 232 | 307 | 331 | 223 |
| 6f | CH2CO2C(CH3)3 | 208 | 247 | 292 | 326 | 238 |
All the synthesized ILs exhibited good thermal stability with high decomposition temperatures. Generally, ILs bearing imidazolium cations exhibited higher thermal stability compared to those with benzimidazolium, Fig. 3 and 4 demonstrate their thermogravimetric analysis traces, respectively. Further, the ILs containing cyanide or ethanolic functional side groups (i.e. 5c and 5d) are the most stable with the highest onset decomposition temperature of 294 and 289 °C, respectively. These di-cationic ILs decompose similarly at the first stage (203–270 °C for imidazolium and 200–255 °C for benzimidazolium ILs); subsequently, they have parallel ramps in the decomposition traces, perhaps indicating a similar decomposition mechanism and products.
Generally, imidazolium and benzimidazolium geminal dicationic ILs incorporating unsaturated side groups showed lower thermal stability than their fully saturated analogues.7 Due to the increasing distance between the alkene and nitrogen of the imidazolium ring in 5a and 6a ILs, an increase in their thermal stability is noticed, while the rigidity of alkyne functional groups in 5b and 6b ILs gave rise to a decrease in the stability (propargyl vs. allyl).83
TGA thermograms of both IL types reveal three main weight loss regions. The first region at a temperature range of 50 to 200 °C is due to the evaporation of physically weak and chemically strong bound water. The weight loss of the ILs in this range is about 5–8 wt% reflecting an acceptable limit of water content. The second transition region at around 210–500 °C is due to the structural degradation of the ILs with 50–70% total weight loss within these ranges of the decomposition temperatures. The third stage weight loss occurred above 500 °C, probably due to the cleavage of the backbone of the ILs where the total weight loss in this stage was ∼20% at 900 °C. The decomposition of the ILs was almost complete at around 900 °C and no further weight loss was observed after that. Compared to many traditional mono- and symmetric dicationic imidazolium-based ILs,6,7,9 the prepared geminal dicationic ILs showed a significant high thermal stability, e.g., thermal stabilities ranging from 145, 185, 257 to 300 °C were recorded for 1-butyl-3-methylimidazolium chloride, 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide, 1,5-bis-(3-(2-ethanolyl)-imidazol-1-iumyl)pentane bis(trifluoromethylsulfonyl)imide, and 1,5-bis-(3-methylimidazol-1-iumyl)pentane-nitrate, respectively. The thermal stability results of the current synthesized ILs support the high decomposition temperatures feature for imidazolium-based dicationic ILs.
Thermal stability of ILs does not strongly rely on the cation structure.77,82,84 Since the prepared geminal dicationic ILs differ only in the cationic substituents of the active side groups on the imidazolium and benzimidazolium rings, minor differences are observed in the decomposition temperatures of these ILs. For example, based on TGA results, Tonset varies between 223 and 294 °C for 6e and 5c, respectively, with approximate weight loss of 10–50%.
3. Experimental section
3.1. General
Allyl bromide (99%), propargyl bromide solution (80% wt with toluene), chloroacetonitrile (99%), 2-bromoethanol (95%), ethyl bromoacetate (98%) and tert-butyl bromoacetate (98%) were purchased from Aldrich and used without further purification. All ILs were kept in the fridge (5 °C) and freezer (−18 °C) for further evaluation of their properties. General grade solvents were purchased from commercial suppliers and used without further purification. The synthesis of compounds 2, 3 and 4 were described and reported in previous work.46 The IR spectra were obtained with a Perkin Elmer 400 Fourier Transform Infrared (FTIR) spectrometer. Both the 1H and 13C-NMR spectra were recorded on a Jeol Lambda and ECA-DELTA as well as Bruker spectrometers at 400 MHz while 19F-NMR was recorded using Bruker spectrometers 400 MHz. High-resolution mass spectra were recorded on an Agilent Technologies 6530 Accurate Q-TOF LC-MS system, applying DMSO–MeOH eluents for IL sample compounds while an Agilent 5975 system for EI/MS (Mass Spectra Service Centre of the National University of Singapore) for the rest of the compounds. Thermogravimetric analysis (TGA) measurements were performed using a Perkin Elmer TGA4000 based on a heating rate of 10 °C min−1 under nitrogen atmosphere. Thin layer chromatography was carried out on pre-coated silica gel plates (0.25 mm, 20 × 20 cm, 60F254, E. Merck).3.2. Synthesis of 5a–f and 6a–f
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)Ar, 1561, 1493 (C
N)Ar, 1561, 1493 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)Ar, 1336, 1156 (O
C)Ar, 1336, 1156 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.43 (bt∼s, 2H, C–HImidazole), 7.94 (t, J = 1.71 Hz, 2H, C–HImidazole), 7.75 (t, J = 1.71 Hz, 2H, C–HImidazole), 7.65 (d, J = 8.05 Hz, 2H, C–HAr), 7.40 (d, J = 8.05 Hz, 2H, C–HAr), 6.08–5.98 (m, 2H, C–HAllyl), 5.36 (d, J = 1.22 Hz, 1H, C–H(1a)Allyl), 5.34 (d, J = 1.22 Hz, 1H, C–H(1b)Allyl), 5.33 (d, J = 1.22 Hz, 1H, C–H(2a)Allyl), 5.29 (d, J = 1.22 Hz, 1H, C–H(2b)Allyl), 4.88 (d, J = 5.85 Hz, 4H, 2 × (α-CH2)Allyl), 4.50 (t, J = 6.59 Hz, 4H, 2 × CH2–N), 3.71 (t, J = 6.34 Hz, 4H, 2 × CH2–N), 2.39 (s, 3H, (CH3)Ts); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 143.89 (CAr–S), 136.59 (2 × CHImidazole), 134.89 (CAr–CH3), 131.58 (2 × (CH)Allyl), 129.95 (2 × CHAr), 126.98 (2 × CHImidazole), 123.05 (2 × CHAr), 122.30 (2 × CHImidazole), 120.10 (2 × (CH2)Allyl), 50.80 (2 × (α-CH2)Allyl), 47.90 (2 × CH2–N), 47.12 (2 × CH2–NAr), 20.97 (CH3)Ts; HRMS: m/z, [M+2 − H]–2Br− calcd for C23H30N5O2S3+: 440.2120, found: 440.2126.
O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.43 (bt∼s, 2H, C–HImidazole), 7.94 (t, J = 1.71 Hz, 2H, C–HImidazole), 7.75 (t, J = 1.71 Hz, 2H, C–HImidazole), 7.65 (d, J = 8.05 Hz, 2H, C–HAr), 7.40 (d, J = 8.05 Hz, 2H, C–HAr), 6.08–5.98 (m, 2H, C–HAllyl), 5.36 (d, J = 1.22 Hz, 1H, C–H(1a)Allyl), 5.34 (d, J = 1.22 Hz, 1H, C–H(1b)Allyl), 5.33 (d, J = 1.22 Hz, 1H, C–H(2a)Allyl), 5.29 (d, J = 1.22 Hz, 1H, C–H(2b)Allyl), 4.88 (d, J = 5.85 Hz, 4H, 2 × (α-CH2)Allyl), 4.50 (t, J = 6.59 Hz, 4H, 2 × CH2–N), 3.71 (t, J = 6.34 Hz, 4H, 2 × CH2–N), 2.39 (s, 3H, (CH3)Ts); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 143.89 (CAr–S), 136.59 (2 × CHImidazole), 134.89 (CAr–CH3), 131.58 (2 × (CH)Allyl), 129.95 (2 × CHAr), 126.98 (2 × CHImidazole), 123.05 (2 × CHAr), 122.30 (2 × CHImidazole), 120.10 (2 × (CH2)Allyl), 50.80 (2 × (α-CH2)Allyl), 47.90 (2 × CH2–N), 47.12 (2 × CH2–NAr), 20.97 (CH3)Ts; HRMS: m/z, [M+2 − H]–2Br− calcd for C23H30N5O2S3+: 440.2120, found: 440.2126.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C), 1613 (C
C), 1613 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)Ar, 1562, 1486 (C
N)Ar, 1562, 1486 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)Ar, 1331, 1154 (O
C)Ar, 1331, 1154 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.40 (bt∼s, 2H, C–HImidazole), 7.89 (t, J = 1.81 Hz, 2H, C–HImidazole), 7.80 (t, J = 1.81 Hz, 2H, C–HImidazole), 7.63 (d, J = 8.15 Hz, 2H, C–HAr), 7.40 (d, J = 8.15 Hz, 2H, C–HAr), 5.23 (d, J = 2.72 Hz, 4H, 2 × (α-CH2)Propargyl), 4.48 (t, J = 6.34 Hz, 4H, 2 × CH2–NAr), 3.88 (t, J = 2.72 Hz, 2H, (C–H)Propargyl), 3.69 (t, J = 6.34 Hz, 4H, 2 × CH2–N), 2.40 (s, 3H, (CH3)Ts); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 144.04 (CAr–S), 136.56 (2 × CHImidazole), 134.87 (CAr–CH3), 130.01 (2 × CHAr), 127.02 (2 × CHImidazole), 123.33 (2 × CHAr), 122.18 (2 × CHImidazole), 79.21 (2 × CPropargyl), 75.94 (2 × CHPropargyl), 47.72 (2 × CH2–N), 47.23 (2 × CH2–NAr), 38.69 (2 × (α-CH2)Propargyl), 21.03 (CH3)Ts; HRMS: m/z, [M+2 − H]–2Br− calcd for C23H25N5O2S3+: 436.1807, found: 436.1810.
O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.40 (bt∼s, 2H, C–HImidazole), 7.89 (t, J = 1.81 Hz, 2H, C–HImidazole), 7.80 (t, J = 1.81 Hz, 2H, C–HImidazole), 7.63 (d, J = 8.15 Hz, 2H, C–HAr), 7.40 (d, J = 8.15 Hz, 2H, C–HAr), 5.23 (d, J = 2.72 Hz, 4H, 2 × (α-CH2)Propargyl), 4.48 (t, J = 6.34 Hz, 4H, 2 × CH2–NAr), 3.88 (t, J = 2.72 Hz, 2H, (C–H)Propargyl), 3.69 (t, J = 6.34 Hz, 4H, 2 × CH2–N), 2.40 (s, 3H, (CH3)Ts); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 144.04 (CAr–S), 136.56 (2 × CHImidazole), 134.87 (CAr–CH3), 130.01 (2 × CHAr), 127.02 (2 × CHImidazole), 123.33 (2 × CHAr), 122.18 (2 × CHImidazole), 79.21 (2 × CPropargyl), 75.94 (2 × CHPropargyl), 47.72 (2 × CH2–N), 47.23 (2 × CH2–NAr), 38.69 (2 × (α-CH2)Propargyl), 21.03 (CH3)Ts; HRMS: m/z, [M+2 − H]–2Br− calcd for C23H25N5O2S3+: 436.1807, found: 436.1810.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1629, 1596 (C
N), 1629, 1596 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)Ar, 1563, 1493 (C
N)Ar, 1563, 1493 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)Ar, 1336, 1155 (O
C)Ar, 1336, 1155 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.88 (bt∼s, 2H, C–HImidazole), 8.17 (t, J = 1.83 Hz, 2H, C–HImidazole), 8.02 (t, J = 1.83 Hz, 2H, C–HImidazole), 7.67 (d, J = 8.24 Hz, 2H, C–HAr), 7.39 (d, J = 8.24 Hz, 2H, C–HAr), 5.90 (s, 4H, 2 × (α-CH2)), 4.61 (t, J = 6.10 Hz, 4H, 2 × CH2–NAr), 3.71 (t, J = 6.10 Hz, 4H, 2 × CH2–N), 2.38 (s, 3H, (CH3)Ts); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 144.03 (CAr–S), 137.90 (2 × CHImidazole), 134.56 (CAr–CH3), 130.00 (2 × CHAr), 127.16 (2 × CHAr), 123.67 (2 × CHImidazole), 122.49 (2 × CHImidazole), 114.73 (2 × CN), 48.20 (2 × CH2–N), 47.58 (2 × CH2–NAr), 36.79 (2 × (α-CH2)), 21.02 (CH3)Ts; HRMS: m/z, [M+2 − H]–2Cl− calcd for C21H24N7O2S3+: 438.1712, found: 438.1715.
O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.88 (bt∼s, 2H, C–HImidazole), 8.17 (t, J = 1.83 Hz, 2H, C–HImidazole), 8.02 (t, J = 1.83 Hz, 2H, C–HImidazole), 7.67 (d, J = 8.24 Hz, 2H, C–HAr), 7.39 (d, J = 8.24 Hz, 2H, C–HAr), 5.90 (s, 4H, 2 × (α-CH2)), 4.61 (t, J = 6.10 Hz, 4H, 2 × CH2–NAr), 3.71 (t, J = 6.10 Hz, 4H, 2 × CH2–N), 2.38 (s, 3H, (CH3)Ts); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 144.03 (CAr–S), 137.90 (2 × CHImidazole), 134.56 (CAr–CH3), 130.00 (2 × CHAr), 127.16 (2 × CHAr), 123.67 (2 × CHImidazole), 122.49 (2 × CHImidazole), 114.73 (2 × CN), 48.20 (2 × CH2–N), 47.58 (2 × CH2–NAr), 36.79 (2 × (α-CH2)), 21.02 (CH3)Ts; HRMS: m/z, [M+2 − H]–2Cl− calcd for C21H24N7O2S3+: 438.1712, found: 438.1715.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)Ar, 1562, 1493 (C
N)Ar, 1562, 1493 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)Ar, 1335, 1155 (O
C)Ar, 1335, 1155 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1066 (C–O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.25 (bt∼s, 2H, C–HImidazole), 7.80 (t, J = 1.81 Hz, 2H, C–HImidazole), 7.69 (t, J = 1.81 Hz, 2H, C–HImidazole), 7.63 (d, J = 8.15 Hz, 2H, C–HAr), 7.36 (d, J = 8.15 Hz, 2H, C–HAr), 5.16 (bs, 2H, 2 × O–H), 4.41 (t, J = 6.34 Hz, 4H, 2 × CH2–NAr), 4.19 (t, J = 4.98 Hz, 4H, 2 × (α-CH2)), 3.69 (t, J = 4.98 Hz, 4H, 2 × C
O), 1066 (C–O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.25 (bt∼s, 2H, C–HImidazole), 7.80 (t, J = 1.81 Hz, 2H, C–HImidazole), 7.69 (t, J = 1.81 Hz, 2H, C–HImidazole), 7.63 (d, J = 8.15 Hz, 2H, C–HAr), 7.36 (d, J = 8.15 Hz, 2H, C–HAr), 5.16 (bs, 2H, 2 × O–H), 4.41 (t, J = 6.34 Hz, 4H, 2 × CH2–NAr), 4.19 (t, J = 4.98 Hz, 4H, 2 × (α-CH2)), 3.69 (t, J = 4.98 Hz, 4H, 2 × C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–OH), 3.64 (t, overlap, 4H, 2 × CH2–N), 2.35 (s, 3H, (CH3)Ts); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 144.13 (CAr–S), 136.79 (2 × CHImidazole), 134.79 (CAr–CH3), 130.11 (2 × CHAr), 127.17 (2 × CHAr), 122.75 (2 × CHImidazole), 122.67 (2 × CHImidazole), 59.37 (2 ×
2–OH), 3.64 (t, overlap, 4H, 2 × CH2–N), 2.35 (s, 3H, (CH3)Ts); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 144.13 (CAr–S), 136.79 (2 × CHImidazole), 134.79 (CAr–CH3), 130.11 (2 × CHAr), 127.17 (2 × CHAr), 122.75 (2 × CHImidazole), 122.67 (2 × CHImidazole), 59.37 (2 × ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2–OH), 51.76 (2 × (α-CH2)), 48.04 (2 × CH2–N), 47.16 (2 × CH2–NAr), 21.11 (CH3)Ts; HRMS: m/z, [M+2 − H]–2Br− calcd for C21H30N5O4S3+: 448.2019, found: 448.2061.
H2–OH), 51.76 (2 × (α-CH2)), 48.04 (2 × CH2–N), 47.16 (2 × CH2–NAr), 21.11 (CH3)Ts; HRMS: m/z, [M+2 − H]–2Br− calcd for C21H30N5O4S3+: 448.2019, found: 448.2061.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1627, 1596 (C
O), 1627, 1596 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)Ar, 1564, 1493, 1449 (C
N)Ar, 1564, 1493, 1449 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)Ar, 1339, 1156 (O
C)Ar, 1339, 1156 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1088 (C–O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.40 (bt∼s, 2H, C–HImidazole), 7.95 (t, J = 1.71 Hz, 2H, C–HImidazole), 7.80 (t, J = 1.71 Hz, 2H, C–HImidazole), 7.68 (d, J = 8.29 Hz, 2H, C–HAr), 7.41 (d, J = 8.05 Hz, 2H, C–HAr), 5.34 (s, 4H, 2 × (α-CH2)), 4.53 (t, J = 6.34 Hz, 4H, 2 × CH2–NAr), 4.20 (q, J = 7.07 Hz, 4H, 2 × O–CH2–), 3.67 (t, J = 6.34 Hz, 4H, 2 × CH2–N), 2.40 (s, 3H, (CH3)Ts), 1.23 (t, J = 7.07 Hz, 6H, 2 × (–CH3)); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 166.69 (2 × C
O), 1088 (C–O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.40 (bt∼s, 2H, C–HImidazole), 7.95 (t, J = 1.71 Hz, 2H, C–HImidazole), 7.80 (t, J = 1.71 Hz, 2H, C–HImidazole), 7.68 (d, J = 8.29 Hz, 2H, C–HAr), 7.41 (d, J = 8.05 Hz, 2H, C–HAr), 5.34 (s, 4H, 2 × (α-CH2)), 4.53 (t, J = 6.34 Hz, 4H, 2 × CH2–NAr), 4.20 (q, J = 7.07 Hz, 4H, 2 × O–CH2–), 3.67 (t, J = 6.34 Hz, 4H, 2 × CH2–N), 2.40 (s, 3H, (CH3)Ts), 1.23 (t, J = 7.07 Hz, 6H, 2 × (–CH3)); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 166.69 (2 × C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 144.00 (CAr–S), 137.63 (2 × CHImidazole), 134.38 (CAr–CH3), 129.98 (2 × CHAr), 127.16 (2 × CHImidazole), 123.65 (2 × CHAr), 122.55 (2 × CHImidazole), 61.86 (2 ×
O), 144.00 (CAr–S), 137.63 (2 × CHImidazole), 134.38 (CAr–CH3), 129.98 (2 × CHAr), 127.16 (2 × CHImidazole), 123.65 (2 × CHAr), 122.55 (2 × CHImidazole), 61.86 (2 × ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2–O), 49.57 (2 × (α-CH2)), 48.16 (2 × CH2–N), 47.43 (2 × CH2–NAr), 20.98 (CH3)Ts, 13.92 (2 × (CH3)); HRMS: m/z, [M+2 − H]–2Br− calcd for C25H34N5O6S3+: 532.2230, found: 532.2234.
H2–O), 49.57 (2 × (α-CH2)), 48.16 (2 × CH2–N), 47.43 (2 × CH2–NAr), 20.98 (CH3)Ts, 13.92 (2 × (CH3)); HRMS: m/z, [M+2 − H]–2Br− calcd for C25H34N5O6S3+: 532.2230, found: 532.2234.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) 1597 (C
O) 1597 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)Ar, 1566, 1443 (C
N)Ar, 1566, 1443 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)Ar, 1364, 1155 (O
C)Ar, 1364, 1155 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1042 (C–O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.35 (bt∼s, 2H, C–HImidazole), 7.90 (t, J = 1.71 Hz, 2H, C–HImidazole), 7.76 (t, J = 1.71 Hz, 2H, C–HImidazole), 7.68 (d, J = 8.29 Hz, 2H, C–HAr), 7.41 (d, J = 8.29 Hz, 2H, C–HAr), 5.22 (s, 4H, 2 × (α-CH2)), 4.51 (t, J = 6.34 Hz, 4H, 2 × CH2–NAr), 3.66 (t, J = 6.34 Hz, 4H, 2 × CH2–N), 2.40 (s, 3H, (–CH3)Ts), 1.45 (s, 18H, 6 × (–CH3)); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 165.71 (2 × C
O), 1042 (C–O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.35 (bt∼s, 2H, C–HImidazole), 7.90 (t, J = 1.71 Hz, 2H, C–HImidazole), 7.76 (t, J = 1.71 Hz, 2H, C–HImidazole), 7.68 (d, J = 8.29 Hz, 2H, C–HAr), 7.41 (d, J = 8.29 Hz, 2H, C–HAr), 5.22 (s, 4H, 2 × (α-CH2)), 4.51 (t, J = 6.34 Hz, 4H, 2 × CH2–NAr), 3.66 (t, J = 6.34 Hz, 4H, 2 × CH2–N), 2.40 (s, 3H, (–CH3)Ts), 1.45 (s, 18H, 6 × (–CH3)); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 165.71 (2 × C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 144.06 (CAr–S), 137.66 (2 × CHImidazole), 134.46 (CAr–CH3), 130.02 (2 × CHAr), 127.18 (2 × CHAr), 123.70 (2 × CHImidazole), 122.49 (2 × CHImidazole), 83.06 (2 × C), 50.04 (2 × (α-CH2)), 48.09 (2 × CH2–N), 47.37 (2 × CH2–NAr), 27.64 (6 × CH3), 21.02 (CH3)Ts; HRMS: m/z, [M+2 − H]–2Br− calcd for C29H42N5O6S3+: 588.2856, found: 588.2913.
O), 144.06 (CAr–S), 137.66 (2 × CHImidazole), 134.46 (CAr–CH3), 130.02 (2 × CHAr), 127.18 (2 × CHAr), 123.70 (2 × CHImidazole), 122.49 (2 × CHImidazole), 83.06 (2 × C), 50.04 (2 × (α-CH2)), 48.09 (2 × CH2–N), 47.37 (2 × CH2–NAr), 27.64 (6 × CH3), 21.02 (CH3)Ts; HRMS: m/z, [M+2 − H]–2Br− calcd for C29H42N5O6S3+: 588.2856, found: 588.2913.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)Ar, 1562, 1485 (C
N)Ar, 1562, 1485 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)Ar, 1331, 1154 (O
C)Ar, 1331, 1154 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 10.09 (s, 2H, C–HBImidazole), 8.15–8.13 (m, 2H, C–HBImidazole), 8.00–7.96 (m, 2H, C–HBImidazole), 7.71–7.65 (m, 4H, CHBImidazole), 7.36 (d, J = 8.29 Hz, 2H, C–HAr), 7.11 (d, J = 8.29 Hz, 2H, C–HAr), 6.15–6.05 (m, 2H, C–HAllyl), 5.47 (d, J = 1.22 Hz, 1H, C–H(1a)Allyl), 5.43 (d, J = 1.22 Hz, 1H, C–H(1b)Allyl), 5.40 (d, J = 0.98 Hz, 1H, C–H(2a)Allyl), 5.37 (d, J = 0.98 Hz, 1H, C–H(2b)Allyl), 5.24 (d, J = 5.61 Hz, 4H, 2 × (α-CH2)Allyl), 4.90 (t, J = 6.34 Hz, 4H, 2 × CH2–NAr), 3.96 (t, J = 6.10 Hz, 4H, 2 × CH2–N), 2.27 (s, 3H, (CH3)Ts); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 143.65 (CAr–S), 142.62 (2 × CHBImidazole), 134.97 (CAr–CH3), 131.16 (2 × CBImidazole), 130.77 (2 × (CH)Allyl), 130.75 (2 × CBImidazole), 129.55 (2 × CHBImidazole), 126.67 (2 × CHBImidazole), 126.52 (2 × CHAr), 126.42 (2 × CHAr), 120.47 (2 × (CH2)Allyl), 113.82 (2 × CHBImidazole), 113.60 (2 × CHBImidazole), 48.74 (2 × (α-CH2)Allyl), 46.19 (2 × CH2–N), 44.82 (2 × CH2–NAr), 20.95 (CH3)Ts; HRMS: m/z, [M+2 − H]−2Br− calcd for C31H34N5O2S3+: 540.2433, found: 540.2470.
O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 10.09 (s, 2H, C–HBImidazole), 8.15–8.13 (m, 2H, C–HBImidazole), 8.00–7.96 (m, 2H, C–HBImidazole), 7.71–7.65 (m, 4H, CHBImidazole), 7.36 (d, J = 8.29 Hz, 2H, C–HAr), 7.11 (d, J = 8.29 Hz, 2H, C–HAr), 6.15–6.05 (m, 2H, C–HAllyl), 5.47 (d, J = 1.22 Hz, 1H, C–H(1a)Allyl), 5.43 (d, J = 1.22 Hz, 1H, C–H(1b)Allyl), 5.40 (d, J = 0.98 Hz, 1H, C–H(2a)Allyl), 5.37 (d, J = 0.98 Hz, 1H, C–H(2b)Allyl), 5.24 (d, J = 5.61 Hz, 4H, 2 × (α-CH2)Allyl), 4.90 (t, J = 6.34 Hz, 4H, 2 × CH2–NAr), 3.96 (t, J = 6.10 Hz, 4H, 2 × CH2–N), 2.27 (s, 3H, (CH3)Ts); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 143.65 (CAr–S), 142.62 (2 × CHBImidazole), 134.97 (CAr–CH3), 131.16 (2 × CBImidazole), 130.77 (2 × (CH)Allyl), 130.75 (2 × CBImidazole), 129.55 (2 × CHBImidazole), 126.67 (2 × CHBImidazole), 126.52 (2 × CHAr), 126.42 (2 × CHAr), 120.47 (2 × (CH2)Allyl), 113.82 (2 × CHBImidazole), 113.60 (2 × CHBImidazole), 48.74 (2 × (α-CH2)Allyl), 46.19 (2 × CH2–N), 44.82 (2 × CH2–NAr), 20.95 (CH3)Ts; HRMS: m/z, [M+2 − H]−2Br− calcd for C31H34N5O2S3+: 540.2433, found: 540.2470.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C), 1613, 1596 (C
C), 1613, 1596 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)Ar, 1562, 1486 (C
N)Ar, 1562, 1486 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)Ar, 1331, 1154 (O
C)Ar, 1331, 1154 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1070 (C–O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 10.02 (s, 2H, C–HBImidazole), 8.13–8.09 (m, 2H, C–HBImidazole), 8.05–8.00 (m, 2H, C–HBImidazole), 7.76–7.70 (m, 4H, CHBImidazole), 7.34 (d, J = 8.15 Hz, 2H, C–HAr), 7.10 (d, J = 8.15 Hz, 2H, C–HAr), 5.58 (d, J = 2.72 Hz, 4H, 2 × (α-CH2)Propargyl), 4.87 (t, J = 5.89 Hz, 4H, 2 × CH2–NAr), 3.95 (t, overlap, 4H, 2 × CH2–N), 3.91 (t, J = 2.27 Hz, 2H, (C–H)Propargyl), 2.26 (s, 3H, (CH3)Ts); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 143.78 (CAr–S), 142.46 (2 × CHBImidazole), 134.70 (CAr–CH3), 131.18 (2 × CBImidazole), 130.36 (2 × CBImidazole), 129.61 (2 × CHAr), 126.99 (2 × CHAr), 126.85 (2 × CHBImidazole), 126.45 (2 × CHBImidazole), 113.76 (4 × CHBImidazole), 79.43 (2 × CPropargyl), 75.42 (2 × CHPropargyl), 45.99 (2 × CH2–N), 44.90 (2 × CH2–NAr), 36.77 (2 × (α-CH2)Propargyl), 20.99 (CH3)Ts; HRMS: m/z, [M+2 − H]–2Br− calcd for C31H30N5O2S3+: 536.2120, found: 536.2048.
O), 1070 (C–O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 10.02 (s, 2H, C–HBImidazole), 8.13–8.09 (m, 2H, C–HBImidazole), 8.05–8.00 (m, 2H, C–HBImidazole), 7.76–7.70 (m, 4H, CHBImidazole), 7.34 (d, J = 8.15 Hz, 2H, C–HAr), 7.10 (d, J = 8.15 Hz, 2H, C–HAr), 5.58 (d, J = 2.72 Hz, 4H, 2 × (α-CH2)Propargyl), 4.87 (t, J = 5.89 Hz, 4H, 2 × CH2–NAr), 3.95 (t, overlap, 4H, 2 × CH2–N), 3.91 (t, J = 2.27 Hz, 2H, (C–H)Propargyl), 2.26 (s, 3H, (CH3)Ts); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 143.78 (CAr–S), 142.46 (2 × CHBImidazole), 134.70 (CAr–CH3), 131.18 (2 × CBImidazole), 130.36 (2 × CBImidazole), 129.61 (2 × CHAr), 126.99 (2 × CHAr), 126.85 (2 × CHBImidazole), 126.45 (2 × CHBImidazole), 113.76 (4 × CHBImidazole), 79.43 (2 × CPropargyl), 75.42 (2 × CHPropargyl), 45.99 (2 × CH2–N), 44.90 (2 × CH2–NAr), 36.77 (2 × (α-CH2)Propargyl), 20.99 (CH3)Ts; HRMS: m/z, [M+2 − H]–2Br− calcd for C31H30N5O2S3+: 536.2120, found: 536.2048.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1614, 1596 (C
N), 1614, 1596 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)Ar, 1563, 1487 (C
N)Ar, 1563, 1487 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)Ar, 1329, 1155 (O
C)Ar, 1329, 1155 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 10.31 (s, 2H, C–HBImidazole), 8.19–8.09 (m, 4H, C–HBImidazole), 7.81–7.71 (m, 4H, C–HBImidazole), 7.39 (d, J = 8.15 Hz, 2H, C–HAr), 7.13 (d, J = 8.15 Hz, 2H, C–HAr), 6.13 (s, 2H, (α-CH2)), 4.95 (t, J = 6.34 Hz, 4H, 2 × CH2–NAr), 3.89 (t, J = 6.34 Hz, 4H, 2 × CH2–N), 2.26 (s, 3H, (CH3)Ts); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 143.92 (CAr–S), 143.83 (2 × CHBImidazole), 134.37 (CAr–CH3), 130.95 (2 × CBImidazole), 130.27 (2 × CBImidazole), 129.66 (2 × CHAr), 127.32 (2 × CHAr), 127.24 (2 × CHBImidazole), 126.66 (2 × CHBImidazole), 114.17 (2 × CHBImidazole), 114.05 (2 × CHBImidazole), 113.34 (2 × CN), 46.51 (2 × CH2–N), 45.35 (2 × CH2–NAr), 34.88 (2 × (α-CH2)), 20.96 (CH3)Ts; HRMS: m/z, [M+2 − H]–2Cl− calcd for C29H28N7O2S3+: 538.2025, found: 538.2077.
O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 10.31 (s, 2H, C–HBImidazole), 8.19–8.09 (m, 4H, C–HBImidazole), 7.81–7.71 (m, 4H, C–HBImidazole), 7.39 (d, J = 8.15 Hz, 2H, C–HAr), 7.13 (d, J = 8.15 Hz, 2H, C–HAr), 6.13 (s, 2H, (α-CH2)), 4.95 (t, J = 6.34 Hz, 4H, 2 × CH2–NAr), 3.89 (t, J = 6.34 Hz, 4H, 2 × CH2–N), 2.26 (s, 3H, (CH3)Ts); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 143.92 (CAr–S), 143.83 (2 × CHBImidazole), 134.37 (CAr–CH3), 130.95 (2 × CBImidazole), 130.27 (2 × CBImidazole), 129.66 (2 × CHAr), 127.32 (2 × CHAr), 127.24 (2 × CHBImidazole), 126.66 (2 × CHBImidazole), 114.17 (2 × CHBImidazole), 114.05 (2 × CHBImidazole), 113.34 (2 × CN), 46.51 (2 × CH2–N), 45.35 (2 × CH2–NAr), 34.88 (2 × (α-CH2)), 20.96 (CH3)Ts; HRMS: m/z, [M+2 − H]–2Cl− calcd for C29H28N7O2S3+: 538.2025, found: 538.2077.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)Ar, 1563, 1485 (C
N)Ar, 1563, 1485 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)Ar, 1330, 1154 (O
C)Ar, 1330, 1154 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.85 (s, 2H, C–HBImidazole), 8.09–8.05 (m, 4H, C–HBImidazole), 7.71–7.66 (m, 4H, C–HBImidazole), 7.34 (d, J = 8.15 Hz, 2H, C–HAr), 7.08 (d, J = 8.15 Hz, 2H, C–HAr), 5.22 (bs, 2H, 2 × O–H), 4.84 (t, J = 6.34 Hz, 4H, 2 × CH2–NAr), 4.57 (t, J = 4.98 Hz, 4H, 2 × (α-CH2)), 3.90 (t, J = 6.34 Hz, 4H, 2 × CH2–N), 3.83 (bt∼s, 4H, 2 ×
O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.85 (s, 2H, C–HBImidazole), 8.09–8.05 (m, 4H, C–HBImidazole), 7.71–7.66 (m, 4H, C–HBImidazole), 7.34 (d, J = 8.15 Hz, 2H, C–HAr), 7.08 (d, J = 8.15 Hz, 2H, C–HAr), 5.22 (bs, 2H, 2 × O–H), 4.84 (t, J = 6.34 Hz, 4H, 2 × CH2–NAr), 4.57 (t, J = 4.98 Hz, 4H, 2 × (α-CH2)), 3.90 (t, J = 6.34 Hz, 4H, 2 × CH2–N), 3.83 (bt∼s, 4H, 2 × ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2–OH), 2.27 (s, 3H, (CH3)Ts); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 143.76 (CAr–S), 142.81 (2 × CHBImidazole), 134.72 (CAr–CH3), 131.22 (2 × CBImidazole), 131.02 (2 × CBImidazole), 129.69 (2 × CHAr), 129.59 (2 × CHAr), 126.65 (2 × CHBImidazole), 126.50 (2 × CHBImidazole), 113.99 (2 × CHBImidazole), 113.44 (2 × CHBImidazole), 58.68 (2 × CH2–OH), 49.48 (2 × (α-CH2)), 46.08 (2 × CH2–N), 44.68 (2 × CH2–NAr), 21.05 (CH3)Ts; HRMS: m/z, [M+2 − H]–2Br− calcd for C29H34N5O4S3+: 548.2332, found: 548.2394.
H2–OH), 2.27 (s, 3H, (CH3)Ts); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 143.76 (CAr–S), 142.81 (2 × CHBImidazole), 134.72 (CAr–CH3), 131.22 (2 × CBImidazole), 131.02 (2 × CBImidazole), 129.69 (2 × CHAr), 129.59 (2 × CHAr), 126.65 (2 × CHBImidazole), 126.50 (2 × CHBImidazole), 113.99 (2 × CHBImidazole), 113.44 (2 × CHBImidazole), 58.68 (2 × CH2–OH), 49.48 (2 × (α-CH2)), 46.08 (2 × CH2–N), 44.68 (2 × CH2–NAr), 21.05 (CH3)Ts; HRMS: m/z, [M+2 − H]–2Br− calcd for C29H34N5O4S3+: 548.2332, found: 548.2394.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1614, 1596 (C
O), 1614, 1596 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)Ar, 1564, 1485, (C
N)Ar, 1564, 1485, (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)Ar, 1339, 1155 (O
C)Ar, 1339, 1155 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1088 (C–O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.98 (s, 2H, C–HBImidazole), 8.13–8.10 (m, 2H, C–HBImidazole), 8.06–8.03 (m, 2H, C–HBImidazole), 7.73–7.68 (m, 4H, CHBImidazole), 7.41 (d, J = 8.54 Hz, 2H, C–HAr), 7.12 (d, J = 8.24 Hz, 2H, C–HAr), 5.69 (s, 4H, 2 × (α-CH2)), 4.91 (t, J = 6.10 Hz, 4H, 2 × CH2–NAr), 4.22 (q, J = 7.23 Hz, 4H, 2 × O–CH2–), 3.89 (t, J = 6.10 Hz, 4H, 2 × CH2–N), 2.27 (s, 3H, –(CH3)Ts), 1.24 (t, J = 7.32 Hz, 6H, 2 × (–CH3)); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 166.51 (2 × C
O), 1088 (C–O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.98 (s, 2H, C–HBImidazole), 8.13–8.10 (m, 2H, C–HBImidazole), 8.06–8.03 (m, 2H, C–HBImidazole), 7.73–7.68 (m, 4H, CHBImidazole), 7.41 (d, J = 8.54 Hz, 2H, C–HAr), 7.12 (d, J = 8.24 Hz, 2H, C–HAr), 5.69 (s, 4H, 2 × (α-CH2)), 4.91 (t, J = 6.10 Hz, 4H, 2 × CH2–NAr), 4.22 (q, J = 7.23 Hz, 4H, 2 × O–CH2–), 3.89 (t, J = 6.10 Hz, 4H, 2 × CH2–N), 2.27 (s, 3H, –(CH3)Ts), 1.24 (t, J = 7.32 Hz, 6H, 2 × (–CH3)); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 166.51 (2 × C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 143.89 (CAr–S), 143.53 (2 × CHBImidazole), 134.34 (CAr–CH3), 131.33 (2 × CBImidazole), 130.57 (2 × CBImidazole), 129.64 (2 × CHAr), 126.90 (2 × CHAr), 126.85 (2 × CHBImidazole), 126.69 (2 × CHBImidazole), 113.95 (2 × CHBImidazole), 113.65 (2 × CHBImidazole), 62.08 (2 ×
O), 143.89 (CAr–S), 143.53 (2 × CHBImidazole), 134.34 (CAr–CH3), 131.33 (2 × CBImidazole), 130.57 (2 × CBImidazole), 129.64 (2 × CHAr), 126.90 (2 × CHAr), 126.85 (2 × CHBImidazole), 126.69 (2 × CHBImidazole), 113.95 (2 × CHBImidazole), 113.65 (2 × CHBImidazole), 62.08 (2 × ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2–O), 47.48 (2 × CH2–N), 46.30 (2 × (α-CH2)), 45.19 (2 × CH2–NAr), 20.99 (CH3)Ts, 13.96 (CH3); HRMS: m/z, [M+2 − H]–2Br− calcd for C33H38N5O6S3+: 632.2543, found: 632.2601.
H2–O), 47.48 (2 × CH2–N), 46.30 (2 × (α-CH2)), 45.19 (2 × CH2–NAr), 20.99 (CH3)Ts, 13.96 (CH3); HRMS: m/z, [M+2 − H]–2Br− calcd for C33H38N5O6S3+: 632.2543, found: 632.2601.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1597 (C
O), 1597 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)Ar, 1564, 1488 (C
N)Ar, 1564, 1488 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)Ar, 1364, 1151 (O
C)Ar, 1364, 1151 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1088 (C–O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.95 (s, 2H, C–HBImidazole), 8.12–8.08 (m, 2H, C–HBImidazole), 8.04–8.00 (m, 2H, C–HBImidazole), 7.73–7.68 (m, 4H, CHBImidazole), 7.41 (d, J = 8.15 Hz, 2H, C–HAr), 7.11 (d, J = 8.15 Hz, 2H, C–HAr), 5.58 (s, 4H, 2 × (α-CH2)), 4.90 (t, J = 6.34 Hz, 4H, 2 × CH2–NAr), 3.88 (t, J = 6.12 Hz, 4H, 2 × CH2–N), 2.26 (s, 3H, (-CH3)Ts), 1.43 (s, 18H, 6 × (-CH3)); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 166.51 (2 × C
O), 1088 (C–O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.95 (s, 2H, C–HBImidazole), 8.12–8.08 (m, 2H, C–HBImidazole), 8.04–8.00 (m, 2H, C–HBImidazole), 7.73–7.68 (m, 4H, CHBImidazole), 7.41 (d, J = 8.15 Hz, 2H, C–HAr), 7.11 (d, J = 8.15 Hz, 2H, C–HAr), 5.58 (s, 4H, 2 × (α-CH2)), 4.90 (t, J = 6.34 Hz, 4H, 2 × CH2–NAr), 3.88 (t, J = 6.12 Hz, 4H, 2 × CH2–N), 2.26 (s, 3H, (-CH3)Ts), 1.43 (s, 18H, 6 × (-CH3)); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 166.51 (2 × C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 143.97 (CAr–S), 143.53 (2 × CHBImidazole), 134.42 (CAr–CH3), 131.39 (2 × CBImidazole), 130.60 (2 × CBImidazole), 129.67 (2 × CHAr), 126.96 (2 × CHAr), 126.90 (2 × CHBImidazole), 126.79 (2 × CHBImidazole), 113.92 (2 × CHBImidazole), 113.64 (2 × CHBImidazole), 83.42 (2 × C), 47.97 (2 × (α-CH2)), 46.31 (2 × CH2–N), 45.15 (2 × CH2–NAr), 27.66 (6 × CH3), 21.06 (CH3)Ts; HRMS: m/z, [M+2 − H]–2Br− calcd for C37H46N5O6S3+: 688.3169, found: 688.3217.
O), 143.97 (CAr–S), 143.53 (2 × CHBImidazole), 134.42 (CAr–CH3), 131.39 (2 × CBImidazole), 130.60 (2 × CBImidazole), 129.67 (2 × CHAr), 126.96 (2 × CHAr), 126.90 (2 × CHBImidazole), 126.79 (2 × CHBImidazole), 113.92 (2 × CHBImidazole), 113.64 (2 × CHBImidazole), 83.42 (2 × C), 47.97 (2 × (α-CH2)), 46.31 (2 × CH2–N), 45.15 (2 × CH2–NAr), 27.66 (6 × CH3), 21.06 (CH3)Ts; HRMS: m/z, [M+2 − H]–2Br− calcd for C37H46N5O6S3+: 688.3169, found: 688.3217.3.3. Synthesis of 7d–f, 8a, 8d and 8f
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)Ar, 1548, 1483 (C
N)Ar, 1548, 1483 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)Ar, 1342, 1218 (C–F), 1332, 1151 (O
C)Ar, 1342, 1218 (C–F), 1332, 1151 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1075 (C–O), 1060 (C–O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 8.98 (bt∼s, 2H, C–HImidazole), 7.78 (t, J = 1.81 Hz, 2H, C–HImidazole), 7.67 (t, J = 1.81 Hz, 2H, C–HImidazole), 7.61 (d, J = 8.15 Hz, 2H, C–HAr), 7.35 (d, J = 8.15 Hz, 2H, C–HAr), 5.15 (bs, 2H, 2 × O–H), 4.41 (t, J = 6.34 Hz, 4H, 2 × CH2–NAr), 4.20 (t, J = 4.98 Hz, 4H, 2 × (α-CH2)), 3.68 (t, J = 4.98 Hz, 4H, 2 × CH2–OH), 3.62 (t, 4H, 2 × CH2–N), 2.33 (s, 3H, (CH3)Ts); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 144.62 (CAr–S), 136.92 (2 × CHImidazole), 134.87 (CAr–CH3), 130.35 (2 × CHAr), 129.12 (2 × CHAr), 124.44, 121.22, 118.00, 114.78 (q, J = 322 Hz, CF3), 122.33 (2 × CHImidazole), 121.97 (2 × CHImidazole), 59.31 (2 ×
O), 1075 (C–O), 1060 (C–O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 8.98 (bt∼s, 2H, C–HImidazole), 7.78 (t, J = 1.81 Hz, 2H, C–HImidazole), 7.67 (t, J = 1.81 Hz, 2H, C–HImidazole), 7.61 (d, J = 8.15 Hz, 2H, C–HAr), 7.35 (d, J = 8.15 Hz, 2H, C–HAr), 5.15 (bs, 2H, 2 × O–H), 4.41 (t, J = 6.34 Hz, 4H, 2 × CH2–NAr), 4.20 (t, J = 4.98 Hz, 4H, 2 × (α-CH2)), 3.68 (t, J = 4.98 Hz, 4H, 2 × CH2–OH), 3.62 (t, 4H, 2 × CH2–N), 2.33 (s, 3H, (CH3)Ts); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 144.62 (CAr–S), 136.92 (2 × CHImidazole), 134.87 (CAr–CH3), 130.35 (2 × CHAr), 129.12 (2 × CHAr), 124.44, 121.22, 118.00, 114.78 (q, J = 322 Hz, CF3), 122.33 (2 × CHImidazole), 121.97 (2 × CHImidazole), 59.31 (2 × ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2–OH), 51.90 (2 × (α-CH2)), 48.03 (2 × CH2–N), 47.10 (2 × CH2–NAr), 21.32 (CH3)Ts; 19F (336, MHz) δ ppm: −80.12 (CF3); HRMS: m/z, [M+2 − H]–2NTf2− calcd for C21H30N5O4S3+: 448.2019, found: 448.2068; m/z, [NTf2]− calcd for C2F6NO4S2−: 279.9173, found: 279.9144.
H2–OH), 51.90 (2 × (α-CH2)), 48.03 (2 × CH2–N), 47.10 (2 × CH2–NAr), 21.32 (CH3)Ts; 19F (336, MHz) δ ppm: −80.12 (CF3); HRMS: m/z, [M+2 − H]–2NTf2− calcd for C21H30N5O4S3+: 448.2019, found: 448.2068; m/z, [NTf2]− calcd for C2F6NO4S2−: 279.9173, found: 279.9144.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1626, 1590 (C
O), 1626, 1590 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)Ar, 1560, 1495, 1449 (C
N)Ar, 1560, 1495, 1449 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)Ar, 1352, 1156 (O
C)Ar, 1352, 1156 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1344, 1218 (C–F), 1075 (C–O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.13 (bt∼s, 2H, C–HImidazole), 7.76 (t, J = 1.81 Hz, 2H, C–HImidazole), 7.72 (t, J = 1.81 Hz, 2H, C–HImidazole), 7.65 (d, J = 8.15 Hz, 2H, C–HAr), 7.42 (d, J = 8.15 Hz, 2H, C–HAr), 5.26 (s, 4H, 2 × (α-CH2)), 4.42 (t, J = 6.34 Hz, 4H, 2 × CH2–NAr), 4.22 (q, J = 7.25 Hz, 4H, 2 × O–CH2–), 3.61 (t, J = 6.34 Hz, 4H, 2 × CH2–N), 2.41 (s, 3H, (CH3)Ts), 1.25 (t, J = 7.25 Hz, 6H, 2 × (–CH3)); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 166.83 (2 × C
O), 1344, 1218 (C–F), 1075 (C–O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.13 (bt∼s, 2H, C–HImidazole), 7.76 (t, J = 1.81 Hz, 2H, C–HImidazole), 7.72 (t, J = 1.81 Hz, 2H, C–HImidazole), 7.65 (d, J = 8.15 Hz, 2H, C–HAr), 7.42 (d, J = 8.15 Hz, 2H, C–HAr), 5.26 (s, 4H, 2 × (α-CH2)), 4.42 (t, J = 6.34 Hz, 4H, 2 × CH2–NAr), 4.22 (q, J = 7.25 Hz, 4H, 2 × O–CH2–), 3.61 (t, J = 6.34 Hz, 4H, 2 × CH2–N), 2.41 (s, 3H, (CH3)Ts), 1.25 (t, J = 7.25 Hz, 6H, 2 × (–CH3)); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 166.83 (2 × C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 144.18 (CAr–S), 137.66 (2 × CHImidazole), 134.47 (CAr–CH3), 130.04 (2 × CHAr), 127.16 (2 × CHImidazole), 124.34, 121.10, 117.87, 114.63 (q, J = 322 Hz, CF3), 123.88 (2 × CHAr), 122.61 (2 × CHImidazole), 61.99 (2 ×
O), 144.18 (CAr–S), 137.66 (2 × CHImidazole), 134.47 (CAr–CH3), 130.04 (2 × CHAr), 127.16 (2 × CHImidazole), 124.34, 121.10, 117.87, 114.63 (q, J = 322 Hz, CF3), 123.88 (2 × CHAr), 122.61 (2 × CHImidazole), 61.99 (2 × ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2–O), 49.62 (2 × (α-CH2)), 48.14 (2 × CH2–N), 47.46 (2 × CH2–NAr), 21.00 (CH3)Ts, 13.96 (2 × (CH3)BEA); 19F (336, MHz) δ ppm: −80.00 (CF3); HRMS: m/z, [M+2 − H]–2NTf2− calcd for C25H34N5O6S3+: 532.2230, found: 532.2252; m/z, [NTf2]− calcd for C2F6NO4S2−: 279.9173, found: 279.9205.
H2–O), 49.62 (2 × (α-CH2)), 48.14 (2 × CH2–N), 47.46 (2 × CH2–NAr), 21.00 (CH3)Ts, 13.96 (2 × (CH3)BEA); 19F (336, MHz) δ ppm: −80.00 (CF3); HRMS: m/z, [M+2 − H]–2NTf2− calcd for C25H34N5O6S3+: 532.2230, found: 532.2252; m/z, [NTf2]− calcd for C2F6NO4S2−: 279.9173, found: 279.9205.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1598 (C
O), 1598 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)Ar, 1560, 1466 (C
N)Ar, 1560, 1466 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)Ar, 1360, 1155 (O
C)Ar, 1360, 1155 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1359, 1218 (C–F), 1056 (C–O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.14 (bt∼s, 2H, C–HImidazole), 7.75 (t, J = 1.95 Hz, 2H, C–HImidazole), 7.70 (t, J = 1.91 Hz, 2H, C–HImidazole), 7.65 (d, J = 8.29 Hz, 2H, C–HAr), 7.41 (d, J = 8.29 Hz, 2H, C–HAr), 5.16 (s, 4H, 2 × (α-CH2)), 4.42 (t, J = 6.34 Hz, 4H, 2 × CH2–NAr), 3.61 (t, J = 6.34 Hz, 4H, 2 × CH2–N), 2.41 (s, 3H, (–CH3)Ts), 1.46 (s, 18H, 6 × (–CH3)); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 165.85 (2 × C
O), 1359, 1218 (C–F), 1056 (C–O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.14 (bt∼s, 2H, C–HImidazole), 7.75 (t, J = 1.95 Hz, 2H, C–HImidazole), 7.70 (t, J = 1.91 Hz, 2H, C–HImidazole), 7.65 (d, J = 8.29 Hz, 2H, C–HAr), 7.41 (d, J = 8.29 Hz, 2H, C–HAr), 5.16 (s, 4H, 2 × (α-CH2)), 4.42 (t, J = 6.34 Hz, 4H, 2 × CH2–NAr), 3.61 (t, J = 6.34 Hz, 4H, 2 × CH2–N), 2.41 (s, 3H, (–CH3)Ts), 1.46 (s, 18H, 6 × (–CH3)); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 165.85 (2 × C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 144.22 (CAr–S), 137.68 (2 × CHImidazole), 134.55 (CAr–CH3), 130.09 (2 × CHAr), 127.16 (2 × CHAr), 124.30, 121.08, 117.86, 114.64 (q, J = 322 Hz, CF3), 123.92 (2 × CHImidazole), 122.55 (2 × CHImidazole), 82.97 (2 × CTBE), 50.51 (2 × (α-CH2)), 48.08 (2 × CH2–N), 47.41 (2 × CH2–NAr), 26.94 (6 × CH3), 20.96 (CH3)Ts; 19F (336, MHz) δ ppm: −80.50 (CF3); HRMS: m/z, [M+2 − H]−2NTf2− calcd for C29H42N5O6S3+: 588.2856, found: 588.2919; m/z, [NTf2]− calcd for C2F6NO4S2−: 279.9173, found: 279.9145.
O), 144.22 (CAr–S), 137.68 (2 × CHImidazole), 134.55 (CAr–CH3), 130.09 (2 × CHAr), 127.16 (2 × CHAr), 124.30, 121.08, 117.86, 114.64 (q, J = 322 Hz, CF3), 123.92 (2 × CHImidazole), 122.55 (2 × CHImidazole), 82.97 (2 × CTBE), 50.51 (2 × (α-CH2)), 48.08 (2 × CH2–N), 47.41 (2 × CH2–NAr), 26.94 (6 × CH3), 20.96 (CH3)Ts; 19F (336, MHz) δ ppm: −80.50 (CF3); HRMS: m/z, [M+2 − H]−2NTf2− calcd for C29H42N5O6S3+: 588.2856, found: 588.2919; m/z, [NTf2]− calcd for C2F6NO4S2−: 279.9173, found: 279.9145.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)Ar, 1562, 1485 (C
N)Ar, 1562, 1485 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)Ar, 1342, 1217 (C–F), 1331, 1154 (O
C)Ar, 1342, 1217 (C–F), 1331, 1154 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.74 (s, 2H, C–HBImidazole), 8.08–8.05 (m, 2H, C–HBImidazole), 7.98–7.93 (m, 2H, C–HBImidazole), 7.73–7.66 (m, 4H, CHBImidazole), 7.31 (d, J = 7.70 Hz, 2H, C–HAr), 7.08 (d, J = 7.70 Hz, 2H, C–HAr), 6.12–6.02 (m, 2H, C–HAllyl), 5.44 (d, J = 1.36 Hz, 1H, C–H(1a)Allyl), 5.42 (d, J = 1.36 Hz, 1H, C–H(1b)Allyl), 5.40 (d, J = 1.36 Hz, 1H, C–H(2a)Allyl), 5.38 (d, J = 1.36 Hz, 1H, C–H(2b)Allyl), 5.17 (d, J = 5.89 Hz, 4H, 2 × (α-CH2)Allyl), 4.78 (t, J = 6.34 Hz, 4H, 2 × CH2–NAr), 3.91 (t, J = 6.80 Hz, 4H, 2 × CH2–N), 2.26 (s, 3H, (CH3)Ts); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 143.87 (CAr–S), 142.64 (2 × CHBImidazole), 134.93 (CAr–CH3), 131.29 (2 × CBImidazole), 130.93 (2 × CBImidazole), 130.74 (2 × (CH)Allyl), 129.63 (2 × CHBImidazole), 126.85 (2 × CHBImidazole), 126.74 (2 × CHAr), 126.42 (2 × CHAr), 124.35, 121.15, 117.95, 114.76 (q, J = 322 Hz, CF3), 120.60 (2 × (CH2)Allyl), 113.90 (2 × CHBImidazole), 113.54 (2 × CHBImidazole), 48.87 (2 × (α-CH2)Allyl), 46.08 (2 × CH2–N), 44.72 (2 × CH2–NAr), 20.98 (CH3)Ts; 19F (336, MHz) δ ppm: −80.05 (CF3); HRMS: m/z, [M+2 − H]–2NTf2− calcd for C31H34N5O2S3+: 540.2433, found: 540.2426; m/z, [NTf2]− calcd for C2F6NO4S2−: 279.9173, found: 279.9138.
O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.74 (s, 2H, C–HBImidazole), 8.08–8.05 (m, 2H, C–HBImidazole), 7.98–7.93 (m, 2H, C–HBImidazole), 7.73–7.66 (m, 4H, CHBImidazole), 7.31 (d, J = 7.70 Hz, 2H, C–HAr), 7.08 (d, J = 7.70 Hz, 2H, C–HAr), 6.12–6.02 (m, 2H, C–HAllyl), 5.44 (d, J = 1.36 Hz, 1H, C–H(1a)Allyl), 5.42 (d, J = 1.36 Hz, 1H, C–H(1b)Allyl), 5.40 (d, J = 1.36 Hz, 1H, C–H(2a)Allyl), 5.38 (d, J = 1.36 Hz, 1H, C–H(2b)Allyl), 5.17 (d, J = 5.89 Hz, 4H, 2 × (α-CH2)Allyl), 4.78 (t, J = 6.34 Hz, 4H, 2 × CH2–NAr), 3.91 (t, J = 6.80 Hz, 4H, 2 × CH2–N), 2.26 (s, 3H, (CH3)Ts); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 143.87 (CAr–S), 142.64 (2 × CHBImidazole), 134.93 (CAr–CH3), 131.29 (2 × CBImidazole), 130.93 (2 × CBImidazole), 130.74 (2 × (CH)Allyl), 129.63 (2 × CHBImidazole), 126.85 (2 × CHBImidazole), 126.74 (2 × CHAr), 126.42 (2 × CHAr), 124.35, 121.15, 117.95, 114.76 (q, J = 322 Hz, CF3), 120.60 (2 × (CH2)Allyl), 113.90 (2 × CHBImidazole), 113.54 (2 × CHBImidazole), 48.87 (2 × (α-CH2)Allyl), 46.08 (2 × CH2–N), 44.72 (2 × CH2–NAr), 20.98 (CH3)Ts; 19F (336, MHz) δ ppm: −80.05 (CF3); HRMS: m/z, [M+2 − H]–2NTf2− calcd for C31H34N5O2S3+: 540.2433, found: 540.2426; m/z, [NTf2]− calcd for C2F6NO4S2−: 279.9173, found: 279.9138.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)Ar, 1563, 1485 (C
N)Ar, 1563, 1485 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)Ar, 1344, 1221 (C–F), 1330, 1154 (O
C)Ar, 1344, 1221 (C–F), 1330, 1154 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.72 (s, 2H, C-HBImidazole), 8.07–8.01 (m, 4H, C–HBImidazole), 7.73–7.67 (m, 4H, C–HBImidazole), 7.33 (d, J = 8.31 Hz, 2H, C–HAr), 7.08 (d, J = 8.07 Hz, 2H, C–HAr), 5.20 (bs, 2H, 2 × O–H), 4.79 (t, J = 6.11 Hz, 4H, 2 × CH2–NAr), 4.56 (t, J = 4.98 Hz, 4H, 2 × (α-CH2)), 3.88 (t, J = 6.11 Hz, 4H, 2 × CH2–N), 3.84 (t, J = 4.98 Hz, 4H, 2 × CH2–OH), 2.27 (s, 3H, (CH3)Ts); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 143.82 (CAr–S), 142.83 (2 × CHBImidazole), 134.74 (CAr–CH3), 131.21 (2 × CBImidazole), 131.05 (2 × CBImidazole), 129.92 (2 × CHAr), 129.57 (2 × CHAr), 126.67 (2 × CHBImidazole), 126.48 (2 × CHBImidazole), 124.33, 121.13, 117.93, 114.73 (q, J = 322 Hz, CF3), 113.95 (2 × CHBImidazole), 113.37 (2 × CHBImidazole), 58.62 (2 ×
O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.72 (s, 2H, C-HBImidazole), 8.07–8.01 (m, 4H, C–HBImidazole), 7.73–7.67 (m, 4H, C–HBImidazole), 7.33 (d, J = 8.31 Hz, 2H, C–HAr), 7.08 (d, J = 8.07 Hz, 2H, C–HAr), 5.20 (bs, 2H, 2 × O–H), 4.79 (t, J = 6.11 Hz, 4H, 2 × CH2–NAr), 4.56 (t, J = 4.98 Hz, 4H, 2 × (α-CH2)), 3.88 (t, J = 6.11 Hz, 4H, 2 × CH2–N), 3.84 (t, J = 4.98 Hz, 4H, 2 × CH2–OH), 2.27 (s, 3H, (CH3)Ts); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 143.82 (CAr–S), 142.83 (2 × CHBImidazole), 134.74 (CAr–CH3), 131.21 (2 × CBImidazole), 131.05 (2 × CBImidazole), 129.92 (2 × CHAr), 129.57 (2 × CHAr), 126.67 (2 × CHBImidazole), 126.48 (2 × CHBImidazole), 124.33, 121.13, 117.93, 114.73 (q, J = 322 Hz, CF3), 113.95 (2 × CHBImidazole), 113.37 (2 × CHBImidazole), 58.62 (2 × ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2–OH), 49.48 (2 × (α-CH2)), 46.08 (2 × CH2–N), 44.60 (2 × CH2–NAr), 20.96 (CH3)Ts; 19F (336, MHz) δ ppm: −80.20 (CF3); HRMS: m/z, [M+2 − H]−2NTf2− calcd for C29H34N5O4S3+: 548.2332, found: 548.2290; m/z, [NTf2]− calcd for C2F6NO4S2−: 279.9173, found: 279.9218.
H2–OH), 49.48 (2 × (α-CH2)), 46.08 (2 × CH2–N), 44.60 (2 × CH2–NAr), 20.96 (CH3)Ts; 19F (336, MHz) δ ppm: −80.20 (CF3); HRMS: m/z, [M+2 − H]−2NTf2− calcd for C29H34N5O4S3+: 548.2332, found: 548.2290; m/z, [NTf2]− calcd for C2F6NO4S2−: 279.9173, found: 279.9218.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1597 (C
O), 1597 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)Ar, 1564, 1488 (C
N)Ar, 1564, 1488 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)Ar, 1364, 1151 (O
C)Ar, 1364, 1151 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1354, 1222 (C–F), 1088 (C–O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.76 (s, 2H, C–HBImidazole), 8.05–8.00 (m, 4H, C–HBImidazole), 7.72–7.67 (m, 4H, C–HBImidazole), 7.39 (d, J = 8.07 Hz, 2H, C–HAr), 7.11 (d, J = 8.07 Hz, 2H, C–HAr), 5.51 (s, 4H, 2 × (α-CH2)TBE), 4.82 (t, J = 6.11 Hz, 4H, 2 × CH2–NAr), 3.85 (t, J = 6.11 Hz, 4H, 2 × CH2–N), 2.27 (s, 3H, (–CH3)Ts), 1.89 (s, 18H, 6 × (–CH3)); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 167.97 (2 × C
O), 1354, 1222 (C–F), 1088 (C–O); 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.76 (s, 2H, C–HBImidazole), 8.05–8.00 (m, 4H, C–HBImidazole), 7.72–7.67 (m, 4H, C–HBImidazole), 7.39 (d, J = 8.07 Hz, 2H, C–HAr), 7.11 (d, J = 8.07 Hz, 2H, C–HAr), 5.51 (s, 4H, 2 × (α-CH2)TBE), 4.82 (t, J = 6.11 Hz, 4H, 2 × CH2–NAr), 3.85 (t, J = 6.11 Hz, 4H, 2 × CH2–N), 2.27 (s, 3H, (–CH3)Ts), 1.89 (s, 18H, 6 × (–CH3)); 13C-NMR (100 MHz, DMSO-d6) δ ppm: 167.97 (2 × C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 144.08 (CAr–S), 143.57 (2 × CHBImidazole), 134.41 (CAr–CH3), 131.50 (2 × CBImidazole), 130.63 (2 × CBImidazole), 129.73 (2 × CHAr), 127.04 (2 × CHAr), 126.93 (2 × CHBImidazole), 126.73 (2 × CHBImidazole), 124.24, 121.18, 117.95, 114.71 (q, J = 322, CF3), 113.99 (2 × CHBImidazole), 113.54 (2 × CHBImidazole), 83.57 (2 × C), 47.58 (2 × (α-CH2)), 46.29 (2 × CH2–N), 45.09 (2 × CH2–NAr), 27.25 (6 × CH3), 21.12 (CH3)Ts; 19F (336, MHz) δ ppm: −80.09 (CF3); HRMS: m/z, [M+2 − H]–2NTf2− calcd for C37H46N5O6S3+: 688.3169, found: 688.3222; m/z, [NTf2]− calcd for C2F6NO4S2−: 279.9173, found: 279.9180.
O), 144.08 (CAr–S), 143.57 (2 × CHBImidazole), 134.41 (CAr–CH3), 131.50 (2 × CBImidazole), 130.63 (2 × CBImidazole), 129.73 (2 × CHAr), 127.04 (2 × CHAr), 126.93 (2 × CHBImidazole), 126.73 (2 × CHBImidazole), 124.24, 121.18, 117.95, 114.71 (q, J = 322, CF3), 113.99 (2 × CHBImidazole), 113.54 (2 × CHBImidazole), 83.57 (2 × C), 47.58 (2 × (α-CH2)), 46.29 (2 × CH2–N), 45.09 (2 × CH2–NAr), 27.25 (6 × CH3), 21.12 (CH3)Ts; 19F (336, MHz) δ ppm: −80.09 (CF3); HRMS: m/z, [M+2 − H]–2NTf2− calcd for C37H46N5O6S3+: 688.3169, found: 688.3222; m/z, [NTf2]− calcd for C2F6NO4S2−: 279.9173, found: 279.9180.3.4. Antibacterial evaluation
Ten standard strains of Gram positive and negative bacteria were used to evaluate the antibacterial activities of the synthesized IL compounds; 5a–f and 6a–f. Based on CLSI guidelines,85 the activities were assessed in terms of minimum inhibitory concentrations (MICs) using microbroth dilution assays. The MIC values are given in mg mL−1 and defined as a lowest concentration that inhibits the bacterial strain growth. Gram positive bacteria included: Streptococcus pyogenes ATCC19615, Staphylococcus aureus ATCC 29213, Bacillus subtilis ATCC6051, Rhodococcus ruber ATCC27863, Enterococcus faecalis ATCC 29212, Staphylococcus epidermidis ATCC12228, while Gram negative bacteria included: Escherichia coli ATCC10538, Salmonella typhimurium ATCC14028, Pseudomonas aeruginosa ATCC15442, Acinetobacter calcoaceticus ATCC 23055. These standards strains were obtained from the collection of Biosciences and Biotechnology School, Faculty of Science and Technology, University Kebangsaan, Malaysia.Distilled water was used as negative control to dissolve all the tested ILs in a concentration range of 0.05–0.5 mg mL−1, while, commercial antibiotics amoxicillin and kanamycin were used as a positive control in the same range of concentrations. A loopful of bacterial cells from the nutrient agar plates of stock cultures was inoculated into 100 mL nutrient broth of 250 mL side arm Erlenmeyer flask. They were incubated at 37 °C for 16 h with vigorous shaking. After incubation, the culture was diluted with fresh media to produce an O.D. 600 nm of 0.1. Fifty μL of standardized 18 h incubated bacterial culture were introduced into test tubes containing 5 mL media, followed by the addition of various concentrations of the tested ILs. All assays were performed in triplicate.
3.5. Thermal stability
Thermal stability in terms of decomposition temperatures of the synthesized halogen ILs was evaluated using a thermogravimetric analyser (TGA Perkin Elmer TGA4000, with Pyris 9.1 software). For thermogravimetry measurements, an open alumina crucible with up to 10 mg weight sample was placed on a sample pan with 20 mL min−1 flow-rate of high pure nitrogen at ambient temperature. Consequently, the samples were heated from 35 to 900 °C, at a heating rate of 10 °C min−1 and the weight change was recorded as a function of the heating temperature. The decomposition temperatures are stated in terms of Tstart (the temperature at which the decomposition of the sample starts), T10% and T50% (the temperatures at which a mass loss of 10% and 50%, respectively, is reached), Tpeak (the maximum temperature derivative of the weight change with respect to time), as well as Tonset (the intersection of the zero mass loss baseline and the tangent line through Tpeak).4. Conclusions
Novel sets of halogen and NTf2 di-imidazolium and di-benzimidazolium ILs containing a high rigidity spacer incorporated into the benzenesulfonamide moiety and various active side substituents were successfully prepared. The structures of these di-cationic ILs were confirmed by classical FTIR, NMR, and HRMS techniques. Metathesis of the halogen anion to NTf2 turned all the ILs into clear liquids at room temperature in excellent yield and purity. Both imidazolium and benzimidazolium series of halogen anions were evaluated for thermal stability as well as in vitro antibacterial activities against ten strains of bacteria. The miscibilities of the prepared ILs in both water and common organic solvents are indicated as well. ILs with acetonitrile substituents (i.e. 5c and 6c) on the imidazolium rings displayed the highest bioactivity and onset decomposition temperature among the studied dicationic ILs. However, most of these ILs demonstrated significant activities against both Gram-positive and Gram-negative bacteria compared to commercial antibiotics; amoxicillin and kanamycin beside of their high thermal stability. Generally, ILs bearing imidazolium dications exhibited higher results of antibacterial activity and thermal stability as compared to those with benzimidazolium dications. Surface properties including critical micelle concentration CMC, surface tension γcmc, Krafft temperature and cloud point as well as more physical properties of the synthesized geminal dicationic ILs (e.g., viscosity and fluorescence) will be reported in due course.Acknowledgements
The authors thank the University of Malaya for financial support by High Impact Research Grant UM-MOE UM.C/625/1/HIR/MOE/F00004-21001 from the Ministry of Education Malaysia.Notes and references
- J. H. Davis, Chem. Lett., 2004, 33, 1072–1077 CrossRef CAS.
- P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis, Wiley, 2007, vol. 2 Search PubMed.
- Ionic Liquids Industrial Applications for Green Chemistry, ed. D. R. Robin and R. S. Kenneth, American Chemical Society, 2002 Search PubMed.
- T. Welton, Chem. Rev., 1999, 99, 2071–2084 CrossRef CAS PubMed.
- S. T. Handy, Chem.–Eur. J., 2003, 9, 2938–2944 CrossRef CAS.
- H. Shirota, T. Mandai, H. Fukazawa and T. Kato, J. Chem. Eng. Data, 2011, 56, 2453–2459 CrossRef CAS.
- J. L. Anderson, R. Ding, A. Ellern and D. W. Armstrong, J. Am. Chem. Soc., 2005, 127, 593–604 CrossRef CAS PubMed.
- Y.-S. Ding, M. Zha, J. Zhang and S.-S. Wang, Colloids Surf., A, 2007, 298, 201–205 CrossRef CAS.
- T. Payagala, J. Huang, Z. S. Breitbach, P. S. Sharma and D. W. Armstrong, Chem. Mater., 2007, 19, 5848–5850 CrossRef CAS.
- R. Engel and J. Cohen, Curr. Org. Chem., 2002, 6, 1453–1467 CrossRef CAS.
- S. I. Lall, D. Mancheno, S. Castro, V. Behaj, J. I. Cohen and R. Engel, Chem. Commun., 2000, 2413–2414 RSC.
- S. Lall, V. Behaj, D. Mancheno, R. Casiano, M. Thomas, A. Rikin, J. Gaillard, R. Raju, A. Scumpia, S. Castro, R. Engel and J. L. I. Cohen, Synthesis, 2002, 11, 1530–1540 Search PubMed.
- J. F. Wishart, S. I. Lall-Ramnarine, R. Raju, A. Scumpia, S. Bellevue, R. Ragbir and R. Engel, Radiat. Phys. Chem., 2005, 72, 99–104 CrossRef CAS.
- M. Yoshizawa, K. Ito-Akita and H. Ohno, Electrochim. Acta, 2000, 45, 1617–1621 CrossRef CAS.
- K. Ito, N. Nishina and H. Ohno, Electrochim. Acta, 2000, 45, 1295–1298 CrossRef CAS.
- W. H. Awad, J. W. Gilman, M. Nyden, R. H. Harris Jr, T. E. Sutto, J. Callahan, P. C. Trulove, H. C. DeLong and D. M. Fox, Thermochim. Acta, 2004, 409, 3–11 CrossRef CAS.
- M. E. V. Valkenburg, R. L. Vaughn, M. Williams and J. S. Wilkes, Thermochim. Acta, 2005, 425, 181–188 CrossRef.
- K. Huang, X. Han, X. Zhang and D. Armstrong, Anal. Bioanal. Chem., 2007, 389, 2265–2275 CrossRef CAS PubMed.
- C. Maton, N. De Vos and C. V. Stevens, Chem. Soc. Rev., 2013, 42, 5963–5977 RSC.
- D. Demberelnyamba, K.-S. Kim, S. Choi, S.-Y. Park, H. Lee, C.-J. Kim and I.-D. Yoo, Bioorg. Med. Chem., 2004, 12, 853–857 CrossRef CAS PubMed.
- K. M. Docherty and J. C. F. Kulpa, Green Chem., 2005, 7, 185–189 RSC.
- J. Pernak, J. Rogoża and I. Mirska, Eur. J. Med. Chem., 2001, 36, 313–320 CrossRef CAS PubMed.
- S. Günal, N. Kaloğlu, İ. Özdemir, S. Demir and İ. Özdemir, Inorg. Chem. Commun., 2012, 21, 142–146 CrossRef.
- P. Mester, M. Wagner and P. Rossmanith, Ecotoxicol. Environ. Saf., 2015, 111, 96–101 CrossRef CAS PubMed.
- N. Papo and Y. Shai, Peptides, 2003, 24, 1693–1703 CrossRef CAS PubMed.
- S. Alyar, H. Zengin, N. Özbek and N. Karacan, J. Mol. Struct., 2011, 992, 27–32 CrossRef CAS.
- M. Basanagouda, K. Shivashankar, M. V. Kulkarni, V. P. Rasal, H. Patel, S. S. Mutha and A. A. Mohite, Eur. J. Med. Chem., 2010, 45, 1151–1157 CrossRef CAS PubMed.
- M. Krátký, J. Vinšová, M. Volková, V. Buchta, F. Trejtnar and J. Stolaříková, Eur. J. Med. Chem., 2012, 50, 433–440 CrossRef PubMed.
- M. A. Weidner-Wells and M. J. Macielag, in Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, Inc., 2000 Search PubMed.
- H. G. Aslan, S. Özcan and N. Karacan, Spectrochim. Acta, Part A, 2012, 98, 329–336 CrossRef CAS PubMed.
- S. Bano, K. Javed, S. Ahmad, I. G. Rathish, S. Singh and M. S. Alam, Eur. J. Med. Chem., 2011, 46, 5763–5768 CrossRef CAS PubMed.
- I. G. Rathish, K. Javed, S. Ahmad, S. Bano, M. S. Alam, K. K. Pillai, S. Singh and V. Bagchi, Bioorg. Med. Chem. Lett., 2009, 19, 255–258 CrossRef CAS PubMed.
- F. Carta, L. di Cesare Mannelli, M. Pinard, C. Ghelardini, A. Scozzafava, R. McKenna and C. T. Supuran, Bioorg. Med. Chem., 2015, 23, 1828–1840 CrossRef CAS PubMed.
- I. R. Ezabadi, C. Camoutsis, P. Zoumpoulakis, A. Geronikaki, M. Soković, J. Glamočilija and A. Ćirić, Bioorg. Med. Chem., 2008, 16, 1150–1161 CrossRef CAS PubMed.
- P. Zoumpoulakis, C. Camoutsis, G. Pairas, M. Soković, J. Glamočlija, C. Potamitis and A. Pitsas, Bioorg. Med. Chem., 2012, 20, 1569–1583 CrossRef CAS PubMed.
- M. Kumar, K. Ramasamy, V. Mani, R. K. Mishra, A. B. A. Majeed, E. D. Clercq and B. Narasimhan, Arabian J. Chem., 2014, 7, 396–408 CrossRef CAS.
- Z. Chen, W. Xu, K. Liu, S. Yang, H. Fan, P. S. Bhadury, D.-Y. Huang and Y. Zhang, Molecules, 2010, 15, 9046–9056 CrossRef CAS PubMed.
- M. J. Nieto, F. L. Alovero, R. H. Manzo and M. R. Mazzieri, Eur. J. Med. Chem., 2005, 40, 361–369 CrossRef CAS PubMed.
- H. G. Aslan, S. Özcan and N. Karacan, Spectrochim. Acta, Part A, 2012, 98, 329–336 CrossRef CAS PubMed.
- M. Kumar, B. Narasimhan, P. Kumar, K. Ramasamy, V. Mani, R. K. Mishra and A. B. A. Majeed, Arabian J. Chem., 2014, 7, 436–447 CrossRef CAS.
- O. A. Blatova, A. M. Asiri, Z. M. Al-amshany, M. N. Arshad and V. A. Blatov, New J. Chem., 2014, 38, 4099–4106 RSC.
- A.-N. M. A. Alaghaz, M. E. Zayed, S. A. Alharbi, R. A. A. Ammar and A. Elhenawy, J. Mol. Struct., 2015, 1084, 352–367 CrossRef CAS.
- Ü. Ö. Özdemir, P. Güvenç, E. Şahin and F. Hamurcu, Inorg. Chim. Acta, 2009, 362, 2613–2618 CrossRef.
- M. Mondelli, V. Bruné, G. Borthagaray, J. Ellena, O. R. Nascimento, C. Q. Leite, A. A. Batista and M. H. Torre, J. Inorg. Biochem., 2008, 102, 285–292 CrossRef CAS PubMed.
- Z. H. Chohan, H. A. Shad, M. H. Youssoufi and T. Ben Hadda, Eur. J. Med. Chem., 2010, 45, 2893–2901 CrossRef CAS PubMed.
- N. Al-Mohammed, Y. Alias, Z. Abdullah, R. Shakir, E. Taha and A. Hamid, Molecules, 2013, 18, 11978–11995 CrossRef CAS PubMed.
- M. M. Cecchini, A. Bendjeriou, N. Mnasri, C. Charnay, F. D. Angelis, F. Lamaty, J. Martinez and E. Colacino, New J. Chem., 2014, 38, 6133–6138 RSC.
- H. Li, C. Yu, R. Chen, J. Li and J. Li, Colloids Surf., A, 2012, 395, 116–124 CrossRef CAS.
- A. H. Jadhav and H. Kim, Chem. Eng. J., 2012, 200–202, 264–274 CrossRef CAS.
- Y. S. Ding, M. Zha, J. Zhang and S. S. Wang, Chin. Chem. Lett., 2007, 18, 48–50 CrossRef CAS.
- J.-C. Chang, W.-Y. Ho, I. W. Sun, Y.-L. Tung, M.-C. Tsui, T.-Y. Wu and S.-S. Liang, Tetrahedron, 2010, 66, 6150–6155 CrossRef CAS.
- J. Pitawala, A. Matic, A. Martinelli, P. Jacobsson, V. Koch and F. Croce, J. Phys. Chem. B, 2009, 113, 10607–10610 CrossRef CAS PubMed.
- P. Rajakumar, R. Raja, S. Selvam, R. Rengasamy and S. Nagaraj, Bioorg. Med. Chem. Lett., 2009, 19, 3466–3470 CrossRef CAS PubMed.
- P. Rajakumar, S. Selvam and M. Dhanasekaran, Tetrahedron Lett., 2005, 46, 6127–6130 CrossRef CAS.
- S. A. Forsyth, J. M. Pringle and D. R. MacFarlane, Aust. J. Chem., 2004, 57, 113–119 CrossRef CAS.
- K. R. Seddon, Nat. Mater., 2003, 2, 363–365 CrossRef CAS PubMed.
- J. Homer and M. C. Perry, J. Chem. Soc., Perkin Trans. 2, 1995, 533–536 RSC.
- S. M. Saadeh, Z. Yasseen, F. A. Sharif and H. M. Abu Shawish, Ecotoxicol. Environ. Saf., 2009, 72, 1805–1809 CrossRef CAS PubMed.
- L. C. Branco, J. N. Rosa, J. J. Moura Ramos and C. A. M. Afonso, Chem.–Eur. J., 2002, 8, 3671–3677 CrossRef CAS.
- M. G. Freire, L. M. N. B. F. Santos, A. M. Fernandes, J. A. P. Coutinho and I. M. Marrucho, Fluid Phase Equilib., 2007, 261, 449–454 CrossRef CAS.
- M. G. Freire, C. M. S. S. Neves, S. P. M. Ventura, M. J. Pratas, I. M. Marrucho, J. Oliveira, J. A. P. Coutinho and A. M. Fernandes, Fluid Phase Equilib., 2010, 294, 234–240 CrossRef CAS.
- Z. He, X. Wang, T. Yao, H. Song and S. Yao, Chin. J. Chem. Eng., 2014, 22, 435–446 CrossRef CAS.
- T. Plech, M. Wujec, A. Siwek, U. Kosikowska and A. Malm, Eur. J. Med. Chem., 2011, 46, 241–248 CrossRef CAS PubMed.
- A. Jallapally, D. Addla, P. Yogeeswari, D. Sriram and S. Kantevari, Bioorg. Med. Chem. Lett., 2014, 24, 5520–5524 CrossRef CAS PubMed.
- S.-M. Lee, W.-J. Chang, A.-R. Choi and Y.-M. Koo, Korean J. Chem. Eng., 2005, 22, 687–690 CrossRef CAS.
- J. Pernak, I. Goc and I. Mirska, Green Chem., 2004, 6, 323–329 RSC.
- M. Matzke, S. Stolte, K. Thiele, T. Juffernholz, J. Arning, J. Ranke, U. Welz-Biermann and B. Jastorff, Green Chem., 2007, 9, 1198–1207 RSC.
- J. Pernak, K. Sobaszkiewicz and I. Mirska, Green Chem., 2003, 5, 52–56 RSC.
- J. Ranke, K. Mölter, F. Stock, U. Bottin-Weber, J. Poczobutt, J. Hoffmann, B. Ondruschka, J. Filser and B. Jastorff, Ecotoxicol. Environ. Saf., 2004, 58, 396–404 CrossRef CAS PubMed.
- R. J. Bernot, M. A. Brueseke, M. A. Evans-White and G. A. Lamberti, Environ. Toxicol. Chem., 2005, 24, 87–92 CrossRef CAS PubMed.
- A. Garcia-Lorenzo, E. Tojo, J. Tojo, M. Teijeira, F. J. Rodriguez-Berrocal, M. P. Gonzalez and V. S. Martinez-Zorzano, Green Chem., 2008, 10, 508–516 RSC.
- N. N. Al-Mohammed, R. S. Duali Hussen, Y. Alias and Z. Abdullah, RSC Adv., 2015, 5, 2869–2881 RSC.
- N. N. Al-Mohammed, R. S. Duali Hussen, T. H. Ali, Y. Alias and Z. Abdullah, RSC Adv., 2015, 5, 21865–21876 RSC.
- J. Pernak and P. Chwała, Eur. J. Med. Chem., 2003, 38, 1035–1042 CrossRef CAS PubMed.
- R. J. Bernot, E. E. Kennedy and G. A. Lamberti, Environ. Toxicol. Chem., 2005, 24, 1759–1765 CrossRef CAS PubMed.
- M. Daniel, L. Harry and V. Mai, Guide to Antimicrobials, San Francisco VA Medical Center Infectious Diseases Section, San Francisco, USA, 2012 Search PubMed.
- J. G. Huddleston, A. E. Visser, W. M. Reichert, H. D. Willauer, G. A. Broker and R. D. Rogers, Green Chem., 2001, 3, 156–164 RSC.
- K. J. Baranyai, G. B. Deacon, D. R. MacFarlane, J. M. Pringle and J. L. Scott, Aust. J. Chem., 2004, 57, 145–147 CrossRef CAS.
- C. P. Fredlake, J. M. Crosthwaite, D. G. Hert, S. N. V. K. Aki and J. F. Brennecke, J. Chem. Eng. Data, 2004, 49, 954–964 CrossRef CAS.
- J. M. Crosthwaite, M. J. Muldoon, J. K. Dixon, J. L. Anderson and J. F. Brennecke, J. Chem. Thermodyn., 2005, 37, 559–568 CrossRef CAS.
- K. Noack, P. S. Schulz, N. Paape, J. Kiefer, P. Wasserscheid and A. Leipertz, Phys. Chem. Chem. Phys., 2010, 12, 14153–14161 RSC.
- R. Tao, G. Tamas, L. Xue, S. L. Simon and E. L. Quitevis, J. Chem. Eng. Data, 2014, 59, 2717–2724 CrossRef CAS.
- A. F. Ferreira, P. N. Simões and A. G. M. Ferreira, J. Chem. Thermodyn., 2012, 45, 16–27 CrossRef CAS.
- H. Tokuda, K. Hayamizu, K. Ishii, M. A. B. H. Susan and M. Watanabe, J. Phys. Chem. B, 2005, 109, 6103–6110 CrossRef CAS PubMed.
- M. A. Wikler, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Eighth Edition, 2009 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra13629f |
| This journal is © The Royal Society of Chemistry 2015 |