Ordered porous structure hybrid films generated by breath figures for directional water penetration†
Tianqi Guo,
Keyu Han,
Liping Heng*,
Moyuan Cao and
Lei Jiang
School of Chemistry and Environment, Beihang University, 100191, China. E-mail: henglp@iccas.ac.cn
First published on 13th October 2015
Abstract
A highly ordered open pore honeycomb-structured hybrid film was fabricated by controlling substrate roughness and wettability with the breath figure (BF) method. This composite consists of a porous hydrophobic polyimide (PI) film and a hydrophilic anodic aluminum oxide (AAO) film. The difference between hydrophilic-hydrophobic surfaces yielded an attractive directional water-penetration function. These findings may lead to new applications for honeycomb-structured composites with directional water functionality in fields such as directional drug delivery, biosensors and molecular filtration.
Introduction
In 1994, François et al. reported the preparation of an ordered porous film by the breath figure (BF) process.1 This technique allows for the creation of films with tunable pore sizes for a diverse array of functions related to multiple fields in physical chemistry. Over the past twenty years, efforts surrounding this technique have focused on modifying the parameters used to prepare the ordered porous film, such as the choice of polymer and solvent, casting and environmental conditions, the presence of additives, the type of substrate and substrate wettability;2–5 fabricating patterned and three-dimensional structures within the film;6,7 generating films with specific properties for optoelectronics,8 photocatalytics,9 antireflective coatings,10 hydrophobic surfaces,11 materials requiring high mechanical strength12,13 and surfaces that promote cell adhesion;14–16 and exploring new applications for porous films, such as size-selective separation17 and liquid reprography.18 Although the literature contains many reports of the honeycomb structure formation, the cooperative influences of substrate roughness and wettability on this process remain to be studied. Furthermore, the unidirectional water-penetration aspects of BF films have not been explored.Heterogeneous materials combine multiple components with different physical, chemical and electronic properties to generate new properties.19 Additionally, functionalized surfaces are of both fundamental and technological importance.20 Various elegant chemical and physical methods have been proposed to manufacture anisotropic wetting surfaces by generating a free-energy gradient through chemical modification21–23 and by building asymmetric topologies consisting of grooves,24,25 channels,26,27 or slanted pillars.28,29 Though disordered macroporous heterogeneous surfaces with different affinities were reported by our group in 2012,20 research into this class of materials remains in its infancy. To the best of our knowledge, the development of an open-pore composite with heterogeneous wettability surfaces for directional water penetration has yet to be reported.
A highly ordered open pore honeycomb-structured hybrid film firstly was fabricated by controlling substrate roughness and wettability with the breath figure (BF) method. The film couples hydrophobic porous polyimide (PI) to hydrophilic anodic aluminum oxide (AAO). Using this material, we developed a unidirectional membrane for water, where water can slowly penetrate from the hydrophobic PI side to the hydrophilic AAO side, but the reverse action is blocked. This directional phenomenon is analogous to an electronic diode, i.e., the one-way flow of current through a p–n junction. Additionally, ordered small-pore heterogeneous membranes will have potential applications in the fields of bio-inspired artificial ion-channels, directional drug delivery, biosensors and molecular filtration.
Experimental
Hexagonal AAO membrane treatment
The bare AAO is hydrophilic (Type B). To obtain super-hydrophilic surfaces, AAO was subjected to O2 plasma (200 W) for 3 min (Type A). To prepare hydrophobic surfaces, the AAO was subjected to O2 plasma (200 W) for 2 min, and then placed into a vacuum chamber and exposed to fluoroalkylsilane (FAS) at 80 °C for 1 h (Type C). For superhydrophobic surfaces, AAO was subjected to 5 min of O2 plasma (200 W), followed by FAS treatment at 80 °C for 6 h (Type D).Preparation of the honeycomb-structured surfaces
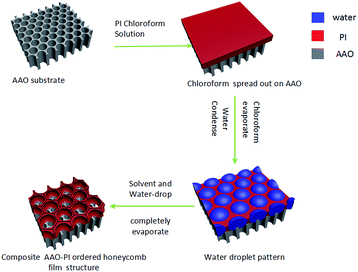
PI was purchased from Sigma-Aldrich; its molecular structure is shown in Fig. S1.† A 1.5 wt% PI solution was prepared by dissolving an appropriate amount of polymer in chloroform, followed by magnetic stirring (1200 rpm) for 30–60 min to completely dissolve the PI. AAO templates with different pore diameters were subjected to various plasma and FAS treatments to achieve surfaces exhibiting different contact angles (CAs). A diagram illustrating the preparation of the honeycomb structure on different AAO membranes is shown in Fig. 1. To obtain honeycomb films, a PI solution (15 μL) was cast onto a treated AAO template at 25 ± 1 °C under a humid atmosphere (relative humidity: 90%).Instruments and characterization
SEM images were obtained using a Quanta FEG250 SEM operated at 10.00 kV. CAs were measured on a JC2000C CA system at ambient temperature. An average value was obtained from at least six different positions on each sample. AFM was performed using a Bruker Dimension Icon with ScanAsyst.Water-penetration on the hybrid film
A small amount of red ink was added to the water for visual enhancement, and it did not influence the wettability of the film. Water droplets were placed on the PI and AAO sides of the hybrid film. Photographs corresponding to the front and back sides of the hybrid film were obtained. The volume of red water was 20 μL in the experiments.Results and discussions
Wettability of water and chloroform on different AAO surfaces
To study the effect of honeycomb pore size on the surface wettability of chloroform and water, we used various treatments to obtain AAO surfaces with different CAs, as shown in Tables 1 and 2. The bare AAO is hydrophilic (Type B). AAO was exposed to O2 plasma and became super-hydrophilic surfaces (Type A). To prepare hydrophobic surfaces, the AAO was subjected to O2 plasma (200 W) for 2 min, and then exposed to fluoroalkylsilane (FAS) (Type C). For superhydrophobic surfaces, AAO was subjected to 5 min of O2 plasma, followed by FAS treatment at 80 °C for 6 h (Type D). Different O2 plasma and FAS treatment time can obtain different hydrophobic molecule modified density.30–32 So different CA can be obtained.| Pore size (nm) | Type A (°) | Type B (°) | Type C (°) | Type D (°) |
|---|---|---|---|---|
| 20–30 | 6.0 ± 1.5 | 25.3 ± 2.2 | 87.5 ± 2.1 | 135.6 ± 1.3 |
| 80–100 | 5.8 ± 1.4 | 29.5 ± 2.7 | 92.1 ± 3.3 | 135.7 ± 1.9 |
| 110–160 | 4.6 ± 1.3 | 30.5 ± 2.3 | 88.1 ± 4.4 | 146.1 ± 1.7 |
| 200–300 | 4.3 ± 1.8 | 31.2 ± 2.2 | 92.9 ± 2.9 | 155.4 ± 4.2 |
| Pore size (nm) | Type A (°) | Type B (°) | Type C (°) | Type D (°) |
|---|---|---|---|---|
| 20–30 | 25.1 ± 2.8 | 24.6 ± 2.8 | 81.2 ± 7.2 | 99.5 ± 3.2 |
| 80–100 | 24.8 ± 3.5 | 20.1 ± 1.7 | 79.8 ± 5.7 | 88.1 ± 4.4 |
| 110–160 | 24.1 ± 1.1 | 19.0 ± 1.2 | 74.9 ± 7.4 | 96.8 ± 2.6 |
| 200–300 | 23.4 ± 2.8 | 18.4 ± 1.5 | 77.4 ± 4.0 | 94.0 ± 4.7 |
For the AAO with a diameter of 20–30 nm, water CAs were 6.0 ± 1.5°, 25.3 ± 2.2°, 87.5 ± 2.1° and 135.6 ± 1.3°, respectively. For the AAO with a diameter of 80–100 nm, the water CAs were 5.8 ± 1.4°, 29.5 ± 2.7°, 92.1 ± 3.3° and 135.7 ± 1.9°, respectively. The water CAs were 4.6 ± 1.3°, 0.5 ± 2.3°, 88.1 ± 4.4° and 146.1 ± 1.7° on the AAO surface with a diameter of 110–160 nm. And water CAs were 4.3 ± 1.8°, 31.2 ± 2.2°, 92.9 ± 2.9° and 155.3 ± 4.2° on the AAO surface with a diameter of 200–300 nm. Notably, only in the case of the AAO surface with a pore diameter of 200–300 nm could all four affinity states (super-hydrophilic, hydrophilic, hydrophobic and superhydrophobic) be observed (Fig. 2). The results indicated that we could regulate surface wettability of the AAO membrane by changing pore sizes. Similarly, we also measured chloroform CAs on different AAO surfaces (Table 2). The results showed that chloroform wettability of the AAO membrane ranged from lyophilic to lyophobic and was tunable through adjustment of the membrane pore size. Variations in wettability, for both water and chloroform, on different AAO surfaces were similar. On the basis of the aforementioned CA data, we deduced that AAO with pore diameters of 200–300 nm exhibited the largest roughness.
Roughness of AAO surface
To confirm our aforementioned inference, we used AFM to systematically measure the roughness of AAO surfaces with different pore sizes. Four types of AAO films with different pore sizes were investigated, as shown in Fig. 3 and S2.† The roughness values ranged from approximately 15 nm for a pore size of 20–30 nm (Fig. 3a and S2a†) to a roughness of approximately 150 nm when the pore size was 200–300 nm. These results indicate that the roughness of AAO membranes increased with increasing pore diameter. An increased pore size was also accompanied by a decrease in pore shape uniformity. In addition, we also studied the effect of plasma treatment on the AAO structure (Fig. S3†). The results showed that AAO surface structures were not affected by the plasma treatment.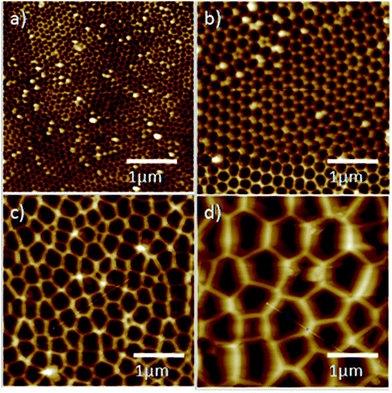 | ||
| Fig. 3 AFM images of AAO membranes with pore sizes of (a) 20–30 nm, (b) 80–100 nm, (c) 110–150 nm and (d) 200–300 nm. | ||
Fabrication of the honeycomb structure surface on different AAO membrane
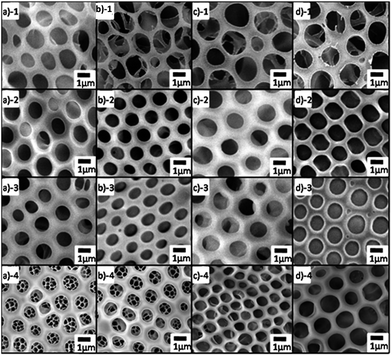
The mechanism for the formation of an BF honeycomb-structured film was first suggested by François et al.;1 Shimomura and co-workers later revealed the process in greater detail.33 Here, we provide only a brief outline. When the water-immiscible organic solvent starts to evaporate, the solution surface temperature decreases. Water vapor from the atmosphere starts to condense. Because the condensation takes place on an unstructured liquid surface, the water droplets have a narrow size distribution. With ongoing evaporation of the organic solvent, these water droplets can grow until the solution becomes too viscous. The droplets of the condensed water self-assemble into a hexagonally ordered array at the air/solution interface. After the evaporation of solvent and water droplets, the well-ordered honeycomb structure is left on the film surface (Fig. 1).Fig. 4 shows SEM images of honeycomb films prepared on different AAO membranes. Fig. 4a-1, b-1, c-1 and d-1 show honeycomb-structured films formed on AAO surfaces possessing the same pore size (20–30 nm) but exhibiting different wettabilities. From this figure, we can see that the honeycomb films have the same pore diameter of approximately 1.0–1.2 μm in Fig. 4a-1 and b-1, whereas the pore sizes in Fig. 4c-1 and d-1 are approximately 1.2–1.3 μm. Fig. 4a-2, b-2, c-2 and d-2 show honeycomb films formed on AAO surfaces with the same pore size (80–100 nm) but with different wettabilities. Honeycomb films formed with a pore diameter of approximately 0.9–1.1 μm in Fig. 4a-2 and b-2 and of approximately 1.0–1.1 μm in Fig. 4c-2 and d-2. Similarly, honeycomb films formed with a diameter of approximately 0.9–1.0 μm in Fig. 4a-3 and b-3 (AAO pore size of 110–150 nm) and of 1.0–1.2 μm in Fig. 4c-3 and d-3. The pore size is 0.8–1.0 μm in Fig. 4a-4 and b-4 (AAO film's pore size of 200–300 nm) and 1.0–1.3 μm in Fig. 4c-4 and d-4; all of the pore wall thicknesses are approximately 400–600 nm. To systematically study the relationship between the pore size of honeycomb film and the wettability of the AAO substrate, we used four type films on AAO with 200–300 nm pore size make a statistics. Fig. S4† shows the pore size distribution histogram of these four types of membrane. The pore sizes are 0.9 ± 0.1 μm, 0.9 ± 0.1 μm, 1.1 ± 0.1 μm and 1.2 ± 0.1 nm corresponding to Fig. 4a-4, b-4, c-4 and d-4, respectively. These results indicate that the honeycomb film's pore size increases as the CA increases. For a given AAO surface wettability, the honeycomb film's pore size remains the same, irrespective of the substrate's pore size. Notably, however, an open pore structure formed on the 200–300 nm AAO lyophilic surfaces (Fig. 4a-4 and b-4). This structure, which should be related to the liquid/substrate wettability and AAO roughness and have applications in numerous fields, was not observed on the other samples.
The formation mechanism of a honeycomb-structured film on different AAO membranes is shown in Fig. 5. Fig. 5a represents a lyophilic surface with small roughness. Upon casting, the polymer solution readily formed a thin liquid film, as evidenced by the small CA between chloroform and the substrate. As the organic solvent began to evaporate, water drops condensed onto the surface of the thin polymer/chloroform layer. After the organic solvent and water evaporated completely, a honeycomb-structured film with closed pores was formed. Fig. 5b represents a lyophilic surface with a large roughness. Unlike the previous scenario, the continuity of the thin polymer/chloroform layer is interrupted by sharp protrusions of the AAO membrane. Under these conditions, the organic solvent and water evaporated leaving behind an open-pore honeycomb film. Fig. 5c and d represent lyophobic surfaces with small and large roughness values, respectively. The polymer solution did not readily wet these surfaces and initially formed a thick liquid film. Therefore, the roughness of the underlying AAO had little influence on the final honeycomb structure, and both substrates produced films with closed pores. Additionally, for a given volume of polymer solution, the poor wetting ability of the PI chloroform on lyophobic surfaces yielded thick liquid layers. The decreased surface area of these layers, with respect to those that formed on lyophilic surfaces, led to a reduced rate of evaporation for chloroform and, ultimately, to larger honeycomb-structured pore sizes.
Directional water-penetration on the honeycomb structure surface
We measured the water CA on both sides of an open-pore hybrid film. Fig. 6 shows photographs of the water droplet shapes on the two surfaces of a hybrid film. The hydrophilic AAO surface (Fig. 6a) and the open-pore hydrophobic PI surface (Fig. 6b) yielded water CAs of 4.3 ± 1.8° and 133.5 ± 2.9°, respectively. These results indicate that the hybrid film exhibited heterogeneous surface wettability, which can be used in the directional permeability of water.20,34–36 The open-pore AAO/PI composite exhibited an interesting unidirectional water-penetration phenomenon. As shown in Fig. 7, water could spontaneously penetrate through the hybrid film from the hydrophobic PI side to the hydrophilic AAO side (Fig. 7a and b). Fig. 7a is a photo of trace amounts of water (dyed red) that remained after being cast onto the PI side. Fig. 7b is a photo of the AAO side of the same composite. The majority of water was observed on the AAO side, as evidenced by the deep-red color. When water was cast onto the hydrophilic AAO side of the composite, the red water droplet spread out horizontally on the surface (Fig. 7c). The PI side of the composite (Fig. 7d) clearly shows the white polymer, with a minimal amount of water. The water droplet clearly could not penetrate the film when first cast onto the AAO side.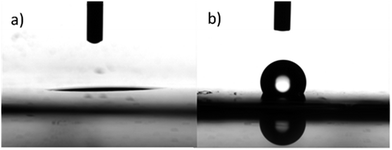 | ||
| Fig. 6 Photographs of the water droplet shape on an open pore hybrid film. The surfaces are (a) the hydrophilic AAO and (b) the hydrophobic porous PI. The diameter of AAO is 200–300 nm. | ||
Mechanism of directional water-penetration through the hybrid film
This directional water penetration through a hybrid porous film is attributed to the film's anisotropic critical breakthrough pressure (Pc). As was recently demonstrated theoretically,36,37 a hydrophilic/hydrophobic membrane possesses a larger Pc for water in the reverse direction because of the coupling effect between local geometric and contact angles. As such, the anisotropy in Pc results in a unidirectional flow of water through the hybrid membrane. This behavior can be preliminarily understood by considering the Laplace pressure of a water droplet and its hydrophilic/hydrophobic interaction with the hybrid film. Air-filled pores on the hydrophobic side form a barrier against water-droplet penetration. When the film is positively aligned (PI side is the top side), a water droplet contacting the hydrophobic side exerts a larger Laplace pressure, which generates a larger driving force for penetration (Fig. 8a). The water droplet with a large Laplace pressure can thus break through the thin hydrophobic layer and reach the underlying hydrophilic layer, whose affinity effectively “pulls” the water across the whole membrane. In contrast, a water droplet contacting the hydrophilic layer tends to spread out, giving a Laplace pressure close to zero (Fig. 8b). This low Laplace pressure limits the driving force for penetration, which is further opposed by the underlying hydrophobic layer. | ||
| Fig. 8 Mechanism of directional water penetration through a heterogeneous hybrid film: (a) water dropped onto the hydrophobic PI side; (b) water dropped onto the hydrophilic AAO side. | ||
Conclusions
Through controlling substrate roughness and wettability, we have successfully used the BF method to produce a hydrophilic/hydrophobic hybrid film with an open pore structure. This composite behaved like a diode, whereby the membrane permitted water to pass through in a single direction. Water can penetrate from the hydrophobic PI side to the hydrophilic AAO side, but the reverse direction is blocked. These findings may assist in the development of new applications for directional water penetration in honeycomb-structured hybrid films.Acknowledgements
This work was supported by the National Research Fund for Fundamental Key Projects (2014CB931802 and 2013CB834705), and the National Natural Science Foundation of China (Grant No. 21207076).Notes and references
- G. Widawski, M. Rawiso and B. François, Nature, 1994, 369, 387 CrossRef CAS PubMed.
- B. de Boer, U. Stalmach, P. F. van Hutten, C. Melzer, V. V. Krasnikov and G. Hadziioannou, Polymer, 2001, 42, 9097–9109 CrossRef CAS.
- L. V. Govor, I. A. Bashmakov, R. Kiebooms, V. Dyakonov and J. Parisi, Adv. Mater., 2001, 13, 588–591 CrossRef CAS.
- C. X. Cheng, Y. Tian, Y. Q. Shi, R. P. Tang and F. Xi, Langmuir, 2005, 21, 6576–6581 CrossRef CAS PubMed.
- T. Nishikawa, J. Nishida, R. Ookura, S. I. Nishimura, V. Scheumann, M. Zizlsperger, R. Lawall, W. Knoll and M. Shimomura, Langmuir, 2000, 16, 1337–1342 CrossRef CAS.
- J. H. Kim, M. Seo and S. Y. Kim, Adv. Mater., 2009, 21, 4130–4133 CrossRef CAS PubMed.
- L. A. Connal, R. Vestberg, C. J. Hawker and G. G. Qiao, Adv. Funct. Mater., 2008, 18, 3706–3714 CrossRef CAS PubMed.
- L. P. Heng, J. Zhai, Y. Zhao, J. J. Xu, X. L. Sheng and L. Jiang, ChemPhysChem, 2006, 7, 2520–2525 CrossRef CAS PubMed.
- K. Kon, C. N. Brauer, K. Hidaka, H. G. Löhmannsröben and O. Karthaus, Langmuir, 2010, 26, 12173–12176 CrossRef CAS PubMed.
- M. S. Park and J. K. Kim, Langmuir, 2005, 21, 11404–11408 CrossRef CAS PubMed.
- H. Yabu, M. Takebayashi, M. Tanaka and M. Shimomura, Langmuir, 2005, 21, 3235–3237 CrossRef CAS PubMed.
- X. Xu, L. P. Heng, X. J. Zhao, J. Ma, L. Lin and L. Jiang, J. Mater. Chem., 2012, 22, 10883–10888 RSC.
- L. P. Heng, B. Wang, M. C. Li, Y. Q. Zhang and L. Jiang, Materials, 2013, 6, 460–482 CrossRef PubMed.
- T. Nishikawa, J. Nishida, R. Ookura, S. I. Nishimura, S. Wada, T. Karino and M. Shimomura, Mater. Sci. Eng., C, 1999, 10, 141–146 CrossRef.
- M. Tanaka, Biochim. Biophys. Acta, Bioenerg., 2011, 1810, 251–258 CrossRef CAS PubMed.
- T. Nishikawa, M. Nonomura, K. Arai, J. Hayashi, T. Sawadaishi, Y. Nishiura, M. Hara and M. Shimomura, Langmuir, 2003, 19, 6193–6201 CrossRef CAS.
- L. S. Wan, J. W. Li, B. B. Ke and Z. K. Xu, J. Am. Chem. Soc., 2011, 134, 95–98 CrossRef PubMed.
- L. P. Heng, J. Li, M. C. Li, D. L. Tian, L. Z. Fan, L. Jiang and B. Z. Tang, Adv. Funct. Mater., 2014, 24, 7241–7248 CrossRef CAS PubMed.
- S. Torquato, Appl. Mech. Rev., 2002, 55, 62 CrossRef.
- J. Wu, N. Wang, L. Wang, H. Dong, Y. Zhao and L. Jiang, Soft Matter, 2012, 8, 5996–5999 RSC.
- S. Daniel, M. K. Chaudhury and J. C. Chen, Science, 2001, 291, 633–636 CrossRef CAS PubMed.
- H. Liu, L. Feng, J. Zhai, L. Jiang and D. B. Zhu, Langmuir, 2004, 20, 5659–5661 CrossRef CAS.
- F. Zhou and W. T. S. Huck, Phys. Chem. Chem. Phys., 2006, 8, 3815–3823 RSC.
- H. Gau, S. Herming haus, P. Lenz and R. Lipowsky, Science, 1999, 283, 46–49 CrossRef CAS.
- W. Li, G. P. Fang, Y. F. Li and G. J. Qiao, J. Phys. Chem. B, 2008, 112, 7234–7243 CrossRef CAS PubMed.
- M. Gleiche, L. F. Chi and H. Fuchs, Nature, 2000, 403, 173–175 CrossRef CAS PubMed.
- L. Zhai, M. C. Berg, F. C. Cebeci, Y. Kim, J. M. Milwid, M. F. Rubner and R. E. Cohen, Nano Lett., 2006, 6, 1213–1217 CrossRef CAS PubMed.
- M. K. Kwak, H. E. Jeong, T. I. Kim, H. Yoon and K. Y. Suh, Soft Matter, 2010, 6, 1849–1857 RSC.
- W. Choi, A. Tuteja, J. M. Mabry, R. E. Cohen and G. H. McKinley, J. Colloid Interface Sci., 2009, 339, 208–216 CrossRef CAS PubMed.
- R. D. Lowe, M. A. Pellow, T. D. P. Stack and C. E. D. Chidsey, Langmuir, 2011, 27, 9928–9935 CrossRef CAS PubMed.
- G. Soliveri, V. Pifferi, R. Annunziata, L. Rimoldi, V. Aina, G. Cerrato, L. Falciola, G. Cappelletti and D. Meroni, J. Phys. Chem. C, 2015, 119, 15390–15400 CAS.
- D. Quéré, Annu. Rev. Mater. Res., 2008, 38, 16.1–16.29 CrossRef.
- N. Maruyama, O. Karthaus, K. Ijiro, M. Shimomura, T. Koito, S. Nishimura, T. Sawadaishi, N. Nishi and S. Tokura, Supramol. Sci., 1998, 5, 331–336 CrossRef CAS.
- H. Zhou, H. Wang, H. Niu and T. Lin, Sci. Rep., 2013, 3, 2964 Search PubMed.
- J. E. Mates, T. M. Schutzius, J. Qin and D. E. Waldroup, ACS Appl. Mater. Interfaces, 2014, 6, 12837–12843 CAS.
- X. Tian, H. Jin, J. Sainio, R. H. A. Ras and O. Ikkala, Adv. Funct. Mater., 2014, 24, 6023–6028 CrossRef CAS PubMed.
- X. Tian, J. Li and X. Wang, Soft Matter, 2012, 8, 2633–2637 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra13627j |
| This journal is © The Royal Society of Chemistry 2015 |