Enhancement of the non-thermal plasma-catalytic system with different zeolites for toluene removal
Rong Huangac,
Meijuan Lua,
Peitao Wanga,
Yangda Chena,
Junliang Wuab,
Mingli Fuab,
Limin Chenab and
Daiqi Ye*ab
aCollege of Environment and Energy, South China University of Technology, Guangzhou 510006, P.R. China. E-mail: cedqye@scut.edu.cn
bGuangdong Provincial Key Laboratory of Atmospheric Environment and Pollution Control of China, Guangzhou 510006, P.R. China
cSouth China Agricultural University, Guangzhou, 510642, P.R. China
First published on 18th August 2015
Abstract
Based on the important effect of catalyst on the plasma-catalytic system, various types of zeolites (5A, HZSM-5, Hβ, HY and Ag/HY) were chosen as catalysts to remove toluene under non-thermal plasma conditions in this work. The results showed that all the zeolites, with or without toluene adsorption abilities, significantly enhanced the toluene removal efficiency in the plasma discharge zone. Moreover, the carbon balance and CO2 selectivity showed the same tendency of Ag/HY > HY > Hβ (HZSM-5) > 5A, which was basically consistent with toluene adsorption ability, while being opposite to the ozone emission. Loading silver on the zeolite greatly decreased organic byproduct emission, and further improved the mineralization of toluene oxidation. At the same time, the intermediates including ring-opening products on the catalyst surface were identified, and the pathways of toluene decomposition were proposed.
1. Introduction
Volatile organic compounds (VOCs) have received increasing attention in recent years because of their harmfulness to human health and the environment. They are regarded as precursors of photochemical smog and dust-haze.1 Although relevant laws and regulations have been legislated in many countries to reduce VOC emission, the terminal treatment technologies are still indispensable for VOC reduction in most countries, especially in developing nations. Some conventional treatment technologies including liquid absorption, active carbon adsorption, thermal incineration and thermal catalytic oxidation were widely used and played important roles in VOC removal, however, each of these technologies has its practical limitations.2–4 Thus, new technologies have been researched and developed, among which plasma-catalysis technology shows promising performance for VOC removal because it combines the advantages of high selectivity from catalysis and rapid reaction from non-thermal plasma (NTP) at room temperature and atmospheric pressure.5In recent years, the technology of NTP combined with catalysts in VOC degradation has been extensively investigated and developed.6–8 In this technology, catalysts play important roles in the improvement of VOC removal. One side, catalysts can prolong the gas retention time and increase the concentration of VOCs in the discharge zone, leading to higher collision probabilities between VOC molecules and active species.3,9 Research showed that the excellent adsorption ability of catalysts could improve the VOC removal in the plasma.10,11 Moreover, adsorption ability was closely related with catalyst pore structure. When the pore diameter of the catalyst is larger than the VOC molecule diameter, the VOC could be adsorbed into the catalyst internal pores. Conversely, when the by-product molecular diameter is greater than the pore diameter, carbon deposition occurs. As various catalysts have different pore diameters, pore diameter has very important effect on both adsorption ability of catalysts and carbon deposition of byproducts. On the other side, in the NTP system, microdischarge might be generated inside the catalyst pores, resulting in more discharge per unit volume and new reactive species formed in the pores of catalysts. These reactive species may promote VOCs decomposition.12 Therefore, it is necessary to study the influence of pore size of catalysts on the removal and mineralization of VOCs by NTP combined with catalysts.
As traditional adsorbents, zeolites have unique micropores, cavities and channels in their frame structures. The size of their micropores range from 3 to 20 Å and it is comparable to the dynamic diameters of some molecules.13 The shape selectivity of zeolites can enable the selective removal of certain gas molecules, and meanwhile zeolites can easily interact with discharge plasma due to a very strong natural electric field within their framework.14 Increasing studies focused on combining plasma with zeolites, and this system has been used for the removal of benzene,9 toluene,15,16 xylene,17 and methane conversion.18,19 These studies paid great attention to VOC removal or conversion by certain zeolite combined in the NTP system, while the relationship between zeolite pore size and the performance of plasma-catalytic oxidation of VOCs is unclear currently.
In this work, toluene (molecule diameter is 5.89 Å),20 which is widely used in many fields of industry, was chosen as a target VOC. And a series of zeolites including 5A, HZSM-5, Hβ and HY were used as catalysts and their pore diameters were 5 Å, 5.5 Å, 6.6 Å and 7.4 Å, respectively. Because silver-based materials are outstanding catalysts in many catalytic oxidation reactions, such as the oxidation of CO, benzyl alcohol and styrene,21 silver loaded zeolite HY was also studied. With each of these zeolites combined in NTP reaction, the toluene removal efficiency, carbon balance and CO2 selectivity were evaluated, and the possible mechanism of toluene oxidation in the NTP-catalytic system was present based on the analysis of byproducts in this study and previous studies in the literature.
2. Experimental
2.1. Experimental setup
The experimental setup is illustrated in Fig. 1 and consists of a dielectric barrier discharge (DBD) plasma reactor, reaction gas supply system, and analytical instrumentation. The reactor was a quartz glass tube with an inner diameter (i.d.) of 10 mm and wall thickness of 1 mm. A stainless steel cylinder (i.d. 2 mm) was used as the ground electrode, while a piece of stainless steel mesh (38 mm × 18 mm) covered around the outside surface of the quartz tube as the high voltage electrode. The effective discharge length and discharge gap were 18 mm and 4 mm, respectively. The reactor was energized by an AC high voltage power supply with the frequency of 2 kHz (CTP-2000k, Corona Laboratory, Nanjing, China). The discharge power was measured by an oscilloscope (TDS1002, Tektronix) and the typical power in the catalyst-plasma reactor was in the range of 3.3–3.7 W. For the plasma driven catalysis process, zeolite catalysts were partly placed in the discharge zone.Experiments were carried out at room temperature and atmospheric pressure. Toluene was produced by passing dry air (carrier gas) through pure liquid toluene kept in an ice/water bath. The air flow rate was adjusted by mass flow controllers. The reaction gas containing 100 ppm toluene passed through the discharge zone and catalyst bed at a rate of 300 ml min−1, and the gas residence time in the catalyst bed was 0.15 s. When toluene adsorption reached saturation, the plasma reaction was started, and meanwhile started to record the concentrations of toluene, CO and CO2 in the outlet gas. Toluene removal efficiency (TRE), carbon balance and CO2 selectivity (CO2%) were defined as follows:
 | (1) |
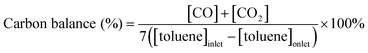 | (2) |
 | (3) |
2.2. Analytical method
Toluene concentration was analyzed on line using a gas chromatograph (GC-2014C, Shimadzu), equipped with two FID detectors. One detector is for organic compounds detection with a 30 m TG-WAXMS capillary column (30 m, 0.32 mm), the other, equipped with a methanizer, is for CO and CO2 analysis with a 5A molecular sieve (2 m, 2 mm) and Poraplot Q column (4 m, 2 mm). The concentration of ozone was monitored by an ozone analyzer (IDEAL-1000, IDEAL instrument).Organic byproducts on the catalysts surface were extracted by CS2 solution (chromatographic grade), and then filtered with an ultrafiltration membrane to get liquid samples. These samples were qualitatively analyzed by a gas chromatograph/mass spectrometry (GCMS-QP2010, Shimadzu) with an Rtx-5MS capillary column. The initial column temperature was 40 °C, maintained for 2 min, and then increased to 220 °C at the rate of 6°C min−1 and maintained for 5 min. MS identification was conducted by NIST 08 databank (NIST/EPA/NIH Mass Spectra Library).
2.3. Catalysts preparation and characterization
Zeolites (5A, HZSM-5, Hβ and HY) were provided by Nan Kai University Catalysts, Co. China. Their basic parameters were listed in Table 1. The zeolites were powders as received, and before use they were compressed into flakes using a hydraulic press, followed by crushing and sieving into particles with the size of 40–60 mesh. The particle size range was selected to limit the pressure drop across the catalyst bed.Silver supported zeolite catalysts were prepared by impregnation method as follows: 0.1575 g AgNO3 was dissolved in 60 ml of ethanol at room temperature, and then 2 g of zeolite powder was added into the above solution with stirring. The mixture was covered with PE film, stirred at room temperature for 16 h, and then put into a 60 °C water bath to undergo the solvent evaporation, followed by calcinations in air at 500 °C for 4 h in a muffle furnace. The resulting catalyst powder was converted to particles with the size of 40–60 mesh by the same method as stated above before use. The theoretical loading amount of silver deduced from chemical analysis was 5 wt%.
To study the effect of the zeolite structure on the catalytic activity, some characterization including X-ray patterns (XRD), scanning electron microscope (SEM) and XPS were measured. XRD patterns were collected by a Bruker D8 diffracometer operated at 30 kV and 30 mA using Cu Kα radiation. Scans were recorded in the range of 2θ between 20 and 80° with a scanning rate of 0.03 s−1. SEM microphotographs were obtained with an electron microscope operating at 5.0 kV (S-3700N, Hitachi, Japan). The valences of Ag on the catalyst surface were analyzed by a Kratos AXIS Ultra DLD spectrometer (Shimadzu, Japan).
3. Results and discussion
3.1. Toluene adsorption on various types of zeolites
Adsorption of toluene was carried out in a fixed adsorption bed. The breakthrough curves (Fig. 2) were obtained at room temperature with a gas hourly space velocity (GHSV) of about 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1. The result showed that only zeolite 5A did not adsorb toluene, and the other zeolites displayed different toluene adsorption abilities in the order of HY > Hβ > HZSM-5. This result was well correlated to the framework structure of the zeolites. The pore diameter of zeolite 5A is less than the mean aerodynamic diameter of toluene molecule. However, zeolites of HY, Hβ and HZSM-5 all have a relatively large 12-ring channel, and the dimensions are 7.4 Å × 7.4 Å, 6.6 Å × 6.7 Å and 6.9 Å × 6.9 Å, respectively,22 which are somewhat larger than the molecular size of toluene. Therefore zeolites HY, Hβ and HZSM-5 could provide enough internal surface to adsorb toluene molecule, while zeolite 5A could not. It was also observed that zeolite HY had better toluene adsorption capacity than HZSM-5 and Hβ, which may be due to its surface acidic characteristics resulted from lower Si/Al ratio than that of HZSM-5 and Hβ.23 The toluene adsorption was greatly improved by silver loading, with Ag/HY showing the best toluene adsorption capability among all the catalysts.
000 h−1. The result showed that only zeolite 5A did not adsorb toluene, and the other zeolites displayed different toluene adsorption abilities in the order of HY > Hβ > HZSM-5. This result was well correlated to the framework structure of the zeolites. The pore diameter of zeolite 5A is less than the mean aerodynamic diameter of toluene molecule. However, zeolites of HY, Hβ and HZSM-5 all have a relatively large 12-ring channel, and the dimensions are 7.4 Å × 7.4 Å, 6.6 Å × 6.7 Å and 6.9 Å × 6.9 Å, respectively,22 which are somewhat larger than the molecular size of toluene. Therefore zeolites HY, Hβ and HZSM-5 could provide enough internal surface to adsorb toluene molecule, while zeolite 5A could not. It was also observed that zeolite HY had better toluene adsorption capacity than HZSM-5 and Hβ, which may be due to its surface acidic characteristics resulted from lower Si/Al ratio than that of HZSM-5 and Hβ.23 The toluene adsorption was greatly improved by silver loading, with Ag/HY showing the best toluene adsorption capability among all the catalysts.
 | ||
| Fig. 2 The breakthrough curves of toluene on zeolites (samples: 200 mg, the inlet concentration of toluene: 100 ppm and flow rate: 300 ml min−1). | ||
In order to find out the relationship between structure and toluene adsorption, Ag/HY was characterized by XRD, XPS and SEM (Fig. 3). Fig. 3a showed that Ag/HY catalyst exhibited the typical peaks of HY, indicating that the structure of the zeolite did not changed during silver loading. Moreover, from the result of SEM analysis (Fig. 3b), it could be noted that some Ag granules appeared and well dispersed on the surface of zeolite HY. In addition, the Ag 3d photoelectron spectra (Fig. 3c) indicated that the Ag species on the surface of catalyst was Ag0, because the Ag 3d5/2 peak at 368.6 eV was assigned to metallic silver.24 As shown in Fig. 3b the particle size of metallic silver granules on the surface of Ag/HY was about 20 nm. It has been proposed that metallic silver nanoparticles could work as adsorption centers for VOCs adsorption,25 and improve the chemical adsorption ability.26 Moreover, Baek suggested that the metals which possessed empty s-orbits and available electrons in the d-orbits could form strong π-complexation bonding,23 and the π-complexation adsorption with toluene on Ag also contributed to the enhancement of adsorption abilities of Ag/HY catalyst. In addition, silver particles could entered into the particular pore channels of support, resulting in the formation of new pore tunnel and new surface area for toluene adsorption.25 As a result, the zeolite Ag/HY owned better toluene adsorption than zeolite HY.
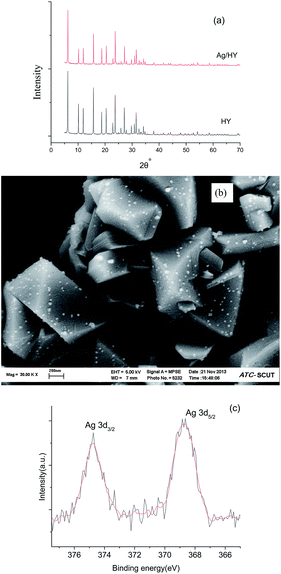 | ||
| Fig. 3 600 dpi in TIF format)[QUESTION MARK]?>XRD patterns (a), SEM image (b) and XPS (c) of Ag/HY catalyst. | ||
3.2. Catalytic activity evaluation
Fig. 4 showed a typical result of toluene removal using the NTP reactor with a representative sample (zeolite Hβ). At the first stage (Stage I), toluene was adsorbed on Hβ. The Hβ was broke through at 42 min and it reached saturation at 192 min. Then, the plasma was turned on (Stage II). During this stage, the concentrations of CO and CO2 immediately increased then quickly decreased, and finally remained at a steady value. The concentration of toluene also showed the same tendency, which indicated that toluene desorbed firstly and then a new adsorption–desorption equilibrium appeared under discharge condition. At the third stage (Stage III), plasma was off again, and the CO and CO2 vanished immediately, whereas the concentration of toluene slowly recovered to influent concentration, which meant that toluene adsorption occurred. Compared to the adsorption stages (Stage I), adsorption capacity of toluene at Stage III showed no significant variation. | ||
| Fig. 4 Typical run of toluene removal in the catalysts combined NTP process (catalyst: zeolite Hβ, toluene inlet concentration: 110 ppm, input discharge power: 3.5 W and catalyst amount: 200 mg). | ||
Catalytic activity of zeolites was determined at the stable states of the discharge reaction (Stage II). Fig. 5 showed the toluene removal efficiency (TRE), carbon balance and CO2 selectivity during this period. The result revealed that whatever type of zeolite was introduced into the discharge zone, toluene removal efficiency was significantly higher than the plasma system without zeolite, and the synergy of NTP and zeolite enhanced the toluene removal efficiency with an increment of approximately 37–56%. Previous researches reported that natural coulombic electric field in the microporous structure of zeolites could strengthen the discharge effect and enhance toluene conversion.27,28 Moreover, in the plasma combined catalysis system, the effect of ozone, adsorption/desorption and the direct interaction of gas phase radicals with the catalyst surface was also related to toluene removal.10,11,29
 | ||
| Fig. 5 TRE, carbon balance and CO2 selectivity in plasma catalytic of toluene (the air flow rate: 300 ml min−1, toluene initial concentration: 100 ppm, and input discharge power: 3.5 W). | ||
In addition, the toluene removal efficiencies of NTP with different zeolites were different. Fig. 5 exhibited that the toluene removal efficiencies in the combined system with zeolites 5A, HZSM-5, HY and Hβ were 79.8%, 80.8%, 85.2% and 98.4%, respectively. Specifically, zeolite 5A did not adsorb toluene, but it exhibited high toluene removal efficiency. This may be attributed to strong collision of electrons and radicals in the micropores, resulting in high discharge effect in unit volume6 and more toluene moleculars impacted by energetic electrons in the gas. Since the other zeolites all had significant toluene adsorption capacities, their toluene conversion ability might depend on both electron impact in the gas phase and adsorption on the surface of zeolite. Furthermore, similar increments in toluene removal efficiency were found in the different zeolites (5A, HZSM-5 and HY), which suggested that the toluene adsorption ability of these zeolites had an insignificant effect on the increase of toluene removal efficiency.
However, toluene adsorption ability greatly influenced the carbon balance and CO2 selectivity. The carbon balance and CO2 selectivity followed the order of HY > Hβ (HZSM-5) > 5A, which was in agreement with toluene adsorption ability (Fig. 2), indicating that the better performance of toluene adsorption, the more complete oxidation of toluene. Moreover, the oxidation improvement was positively proportional to the porosity of the catalysts.30 Compared to zeolites 5A, Hβ and HZSM-5, zeolite HY owned biggest pore diameter that allowed more toluene to transport into the internal pore and provided largest surface area for toluene adsorption. It was well known that the surface of a catalyst can be activated by the plasma and then adsorb active species.31 These active species could ultimately promote toluene oxidation. Thus, the highest toluene mineralization was achieved in the reactor with zeolite HY as catalyst.
In order to further promote the performance of the combined system, Ag was loaded on the surface of zeolite HY and toluene removal efficiency, carbon balance and CO2 selectivity increased from 85.2%, 83.0% and 69.0 to 97.0%, 98.1% and 93.3%, respectively (Fig. 3). For the reason, one side, Ag/HY showed better toluene adsorption ability than zeolite HY (Fig. 2), and according to the analysis above, more adsorbed toluene was completely oxidized. The other side, the enhancement of mineralization will be confirmed by the decrease of byproducts in the section 3.4.
3.3. Byproducts
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36–39 was packed downstream after the plasma zone, i.e. post plasma catalysis system, where the catalysts could not influence the plasma discharge. In both cases, ozone can be decomposed by catalytic reaction, resulting in ozone formation decrease. Therefore the variation of ozone production in the where plasma-catalysis system may be relevant to the way of catalysts being introduced into the system. The decomposition of ozone in the plasma-catalysis system can be expressed as the following reactions:40
36–39 was packed downstream after the plasma zone, i.e. post plasma catalysis system, where the catalysts could not influence the plasma discharge. In both cases, ozone can be decomposed by catalytic reaction, resulting in ozone formation decrease. Therefore the variation of ozone production in the where plasma-catalysis system may be relevant to the way of catalysts being introduced into the system. The decomposition of ozone in the plasma-catalysis system can be expressed as the following reactions:40| O3 + Z → Z–O3 | (R1) |
| Z–O3 → Z–O* + O2 | (R2) |
| Peak number | Retention time (min) | Byproducts |
|---|---|---|
| (1) | 4.601 | Toluene |
| (2) | 6.760 | O-Xylene |
| (3) | 7.834 | Ethane |
| (4) | 8.981 | Benzaldehyde |
| (5) | 9.699 | Heptane |
| (6) | 10.714 | Benzyl alcohol |
| (7) | 11.828 | Benzaldehyde |
| (8) | 13.642 | Benzene |
| (9) | 14.792 | Benzene |
| (10) | 15.191 | Phenol |
| (11) | 16.056 | Dodecane |
| (12) | 21.019 | Benzene |
| (13) | 21.950 | Hexadecane |
| (14) | 23.633 | Pentadecane |
| (15) | 27.979 | Heneicosane |
Compared to the relative abundance of byproducts on the surface of various zeolites, it could be found that there were much fewer productions on the surface of zeolite 5A than on that of other zeolites. This phenomenon was similar with the research of Tao Jiang et al.,28 who conducted the investigation on methane conversion using zeolite A combined with dielectric-barrier discharge and drew a conclusion that zeolite A inhibited the formation of carbon black and plasma polymerized film.28 In addition, zeolite 5A could not adsorb molecules bigger than its pore size in the gas-phase, and only a small amount of deposition of the aerosol byproducts on the external surface. However, zeolites HY, Hβ and HZSM-5 owned excellent toluene adsorption ability and enhanced the conversion of toluene, resulting in more byproducts. Meanwhile, they can serve as reservoirs for gas-phase organic compounds and deposited aerosol due to their particular pore structure and surface characteristics. Therefore, there were more materials appeared on their surfaces than on the surface of zeolite 5A. Furthermore, majority of detected byproducts in bare zeolites reaction were not found when Ag/HY catalysis was combined with NTP reaction in this study, because the metallic silver can decompose ozone and form active oxygen which was beneficial to deep oxidation of the reaction intermediates.42
3.4. Mechanism of toluene decomposition in plasma-catalysis system
The possible pathways of toluene decomposition by NTP combined catalysis were showed in Fig. 8, which based on the hypothesis that toluene was destructed mainly by electron impact and active species (e.g. O3, O and OH) oxidation.35 Generally, the pathway of toluene destruction was closely related to the bond energy of chemical groups. In this work, the average energy of electron in NTP is up to 10 eV,44 and the bond energy of C–H in the methyl group is the smallest one (3.7 eV) compared to other bonds including C–H in aromatic ring (4.3 eV), C–C between methyl group and aromatic ring (4.4 eV), C–C in aromatic ring (5.0–5.3 eV) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in aromatic ring (5.5 eV).45 Therefore, the primary process of toluene oxidation was the breaking of C–H in methyl group. C–H in methyl group could be damaged by high energy electron and abstracted by hydroxyl radical in NTP,43 resulting in the formation of a benzyl radical. Then the benzyl radical could react with oxygen species and formed benzaldehyde and benzyl alcohol. In addition, C–C between methyl group and benzene ring could be broken to form phenyl group, which can bond with H˙, OH˙ and CH3˙ to produce benzene, phenol and o-xylene, respectively. And the ring-containing intermediate products could be further broken down by energetic electron to produce ring-opening intermediates.35 Furthermore, in plasma system, cracking and polymerization reactions could occur simultaneously. As a result, ring-opening intermediates could crack or polymerize to form ethane, heptane, dodecane, hexadecane, pentadecane and heneicosane. Huang H. B. et al. proposed the reaction pathways of toluene decomposition in NTP-catalysis system and only ring-retaining products were detected in the proposed pathways, but the step of ring-opening was pointed out and ring-opening intermediates were finally oxidized to CO2 and H2O.35
C in aromatic ring (5.5 eV).45 Therefore, the primary process of toluene oxidation was the breaking of C–H in methyl group. C–H in methyl group could be damaged by high energy electron and abstracted by hydroxyl radical in NTP,43 resulting in the formation of a benzyl radical. Then the benzyl radical could react with oxygen species and formed benzaldehyde and benzyl alcohol. In addition, C–C between methyl group and benzene ring could be broken to form phenyl group, which can bond with H˙, OH˙ and CH3˙ to produce benzene, phenol and o-xylene, respectively. And the ring-containing intermediate products could be further broken down by energetic electron to produce ring-opening intermediates.35 Furthermore, in plasma system, cracking and polymerization reactions could occur simultaneously. As a result, ring-opening intermediates could crack or polymerize to form ethane, heptane, dodecane, hexadecane, pentadecane and heneicosane. Huang H. B. et al. proposed the reaction pathways of toluene decomposition in NTP-catalysis system and only ring-retaining products were detected in the proposed pathways, but the step of ring-opening was pointed out and ring-opening intermediates were finally oxidized to CO2 and H2O.35
In plasma-catalysis system, homogenous reaction in the gas-phase and heterogeneous reaction on the solid surface of catalysts were considered to be two main reactions for pollutants decomposition. Fig. 4 showed that there were both adsorption and desorption of toluene occured on the surfaces of zeolites during discharge process. So in the process of plasma-catalytic oxidation of toluene, homogeneous and heterogeneous reaction took place together. In the gas phase, toluene destruction mainly depended on the attack of energetic electrons. However, on the interface of gas phase and solid zeolite surface, the process of toluene decomposition was complicated. Compared to the reaction of toluene with atomic oxygen (5.7 × 10−12 cm3 mol−1 s−1), the reaction of ozone with toluene in the gas-phase is very slow (1.2 × 10−20 cm3 mol−1 s−1),36 and plasma-generated ozone alone can not destroy pollutants completely. Therefore, it could be inferred that it was primarily the atomic oxygen species that oxidize toluene. Besides that, from the results of Fig. 2, 5 and 6, the carbon balance and CO2 selectivity followed the order of HY > Hβ (HZSM-5) > 5A which was agreement with toluene adsorption abilities, while opposed with the ozone emission. Therefore, adsorption ability of toluene and ozone play important roles, and it was the adsorbed toluene that reacted with atomic oxygen, which was responsible for the complete oxidation of toluene.
According to the adsorption features of various types of zeolites, toluene decomposition on zeolites could be described as following reactions ((R3)–(R8)):10,46
| C7H8 (g) + e → CO + CO2 + IM | (R3) |
| C7H8 (g) + Z → Z–C7H8* | (R4) |
| Z–C7H8* + Z–O* → CO + CO2 + IM | (R5) |
| Ag + O3 → Ag–O* + O2 | (R6) |
| IM + Ag–O* → CO + CO2 | (R7) |
| CO + Ag–O* → CO2 | (R8) |
4. Conclusion
Zeolites with various pore sizes showed great effects on toluene removal in a NTP reactor and several conclusions could be drew in this work. Firstly, all of the zeolites could improve the toluene removal efficiency, carbon balance and CO2 selectivity of toluene oxidation. Secondly, toluene removal efficiency depended on the discharge effect of NTP and toluene adsorption ability of catalysts, and toluene adsorption ability of catalysts was mainly responsible for the improvement of carbon balance and CO2 selectivity. Lastly, loading silver can further decrease the organic byproducts and enhance mineralization of toluene oxidation.Acknowledgements
The authors would like to acknowledge the financial support for this work by the National Natural Science Foundation of China (No. 50978103, No. U1201231), the National High-tech Research and Development Program of China (No. 2013AA065005), the Fundamental Research Funds for the Central Universities, Science and Technology Program of Guangzhou (No. 201510010164) and the fund of Guangdong Provincial Key Laboratory of Atmospheric Environment and Pollution Control of China.Notes and references
- J. Ma, X. Xu, C. Zhao and P. Yan, Adv. Atmos. Sci., 2012, 29, 1006–1026 CrossRef
.
- W. B. Li, J. X. Wang and H. Gong, Catal. Today, 2009, 148, 81–87 CrossRef CAS PubMed
.
- G. Xiao, W. Xu, R. Wu, M. Ni, C. Du, X. Gao, Z. Luo and K. Cen, Plasma Chem. Plasma Process., 2014, 34, 1033–1065 CrossRef CAS
.
- F. I. Khan and A. K. Ghoshal, J. Loss Prev. Process Ind., 2000, 13, 527–545 CrossRef
.
- H.-H. Kim, Plasma Processes Polym., 2004, 1, 91–110 CrossRef CAS PubMed
.
- F. Holzer, F. D. Kopinke and U. Roland, Plasma Chem. Plasma Process., 2005, 25, 595–611 CrossRef CAS
.
- G. Lombardi, N. Blin-Simiand, F. Jorand, L. Magne, S. Pasquiers, C. Postel and J. R. Vacher, Plasma Chem. Plasma Process., 2007, 27, 414–445 CrossRef CAS
.
- J. Wu, Q. Xia, H. Wang and Z. Li, Appl. Catal., B, 2014, 156–157, 265–272 CrossRef CAS PubMed
.
- A. Ogata, D. Ito, K. Mizuno, S. Kushiyama and T. Yamamoto, IEEE Trans. Ind. Appl., 2001, 37(4), 959–964 CrossRef CAS
.
- Q. H. Trinh, M. S. Gandhi and Y. S. Mok, Jpn. J. Appl. Phys., 2015, 54, 01AG04 CrossRef
.
- M. Lu, R. Huang, J. Wu, M. Fu, L. Chen and D. Ye, Catal. Today, 2015, 242, 274–286 CrossRef CAS PubMed
.
- U. Roland, F. Holzer and F. D. Kopinke, Appl. Catal., B, 2005, 58, 217–226 CrossRef CAS PubMed
.
- H.-H. Kim, Y. Teramoto, T. Sano, N. Negishi and A. Ogata, Appl. Catal., B, 2015, 166–167, 9–17 CrossRef CAS PubMed
.
- C.-j. Liu, J.-x. Wang, K.-l. Yu, B. Eliasson, Q. Xia, B. Xue and Y.-h. Zhang, J. Electrost., 2002, 54, 149–158 CrossRef CAS
.
- S. M. Oh, H. H. Kim, H. Einaga, A. Ogata, S. Futamura and D. W. Park, Thin Solid Films, 2006, 506–507, 418–422 CrossRef CAS PubMed
.
- T. Kuroki, K. Hirai, S. Matsuoka, J. Y. Kim and M. Okubo, IEEE Trans. Ind. Appl., 2011, 47, 1916–1921 CrossRef CAS
.
- T. Kuroki, K. Hirai, R. Kawabata, M. Okubo and T. Yamamoto, IEEE Trans. Ind. Appl., 2010, 46, 672–679 CrossRef CAS
.
- B. Eliasson, C.-j. Liu and U. Kogelschatz, Ind. Eng. Chem. Res., 2000, 39, 1221–1227 CrossRef CAS
.
- K. Zhang, B. Eliasson and U. Kogelschatz, Ind. Eng. Chem. Res., 2002, 41, 1462–1468 CrossRef CAS
.
- C. Y. Chao, C. W. Kwong and K. S. Hui, J. Hazard. Mater., 2007, 143, 118–127 CrossRef CAS PubMed
.
- C. Wen, A. Yin and W.-L. Dai, Appl. Catal., B, 2014, 160–161, 730–741 CrossRef CAS PubMed
.
- J.-H. Park, S. J. Park, I.-S. Nam, G. K. Yeo, J. K. Kil and Y. K. Youn, Microporous Mesoporous Mater., 2007, 101, 264–270 CrossRef CAS PubMed
.
- S.-W. Baek, J.-R. Kim and S.-K. Ihm, Catal. Today, 2004, 93–95, 575–581 CrossRef CAS PubMed
.
- D.-Z. Zhao, X.-S. Li, C. Shi, H.-Y. Fan and A.-M. Zhu, Chem. Eng. Sci., 2011, 66, 3922–3929 CrossRef CAS PubMed
.
- D. Chen, Z. Qu, S. Shen, X. Li, Y. Shi, Y. Wang, Q. Fu and J. Wu, Catal. Today, 2011, 175, 338–345 CrossRef CAS PubMed
.
- D. Chen, Z. Qu, Y. Lv, X. Lu, W. Chen and X. Gao, J. Mol. Catal. A: Chem., 2015, 404–405, 98–105 CrossRef CAS PubMed
.
- B. Raju, E. Reddy, J. Karupplah, P. K. Reddy and C. Subrahmanyam, J. Chem. Sci., 2013, 125, 673–678 CrossRef CAS
.
- T. Jiang, Y. Li and C.-j. Liu, et al., Catal. Today, 2002, 72, 229–235 CrossRef CAS
.
- H.-H. Kim, A. Ogata and S. Futamura, Appl. Catal., B, 2008, 79, 356–367 CrossRef CAS PubMed
.
- U. Roland, F. Holzer and F.-D. Kopinke, Catal. Today, 2002, 73, 315–323 CrossRef CAS
.
- H.-H. Kim, Y.-H. Lee, A. Ogata and S. Futamura, Catal. Commun., 2003, 4, 347–351 CrossRef CAS
.
- L. Chen, X. Zhang, L. Huang and L. Lei, J. Nat. Gas Chem., 2010, 19, 628–637 CrossRef CAS
.
- Y. Sun, L. Zhou, L. Zhang and H. Sui, J. Environ. Sci., 2012, 24, 891–896 CrossRef CAS
.
- Y. Guo, X. Liao, J. He, W. Ou and D. Ye, Catal. Today, 2010, 153, 176–183 CrossRef CAS PubMed
.
- H. Huang, D. Ye, D. Y. C. Leung, F. Feng and X. Guan, J. Mol. Catal. A: Chem., 2011, 336, 87–93 CrossRef CAS PubMed
.
- A. M. Harling, D. J. Glover, J. C. Whitehead and K. Zhang, Appl. Catal., B, 2009, 90, 157–161 CrossRef CAS PubMed
.
- X. Fan, T. L. Zhu, M. Y. Wang and X. M. Li, Chemosphere, 2009, 75, 1301–1306 CrossRef CAS PubMed
.
- J. Jarrige and P. Vervisch, Appl. Catal., B, 2009, 90, 74–82 CrossRef CAS PubMed
.
- T. Zhu, J. Li, W. Liang and Y. Jin, J. Hazard. Mater., 2009, 165, 1258–1260 CrossRef CAS PubMed
.
- M. Magureanu, N. B. Mandache, P. Eloy, E. M. Gaigneaux and V. I. Parvulescu, Appl. Catal., B, 2005, 61, 12–20 CrossRef CAS PubMed
.
- S. Delagrange, L. Pinard and J. Tatibouet, Appl. Catal., B, 2006, 68, 92–98 CrossRef CAS PubMed
.
- H.-H. Kim, M. Sugasawa, H. Hirata, Y. Teramoto, K. Kosuge, N. Negishi and A. Ogata, Plasma Chem. Plasma Process., 2013, 33, 1083–1098 CrossRef CAS
.
- J. Van Durme, J. Dewulf, W. Sysmans, C. Leys and H. Van Langenhove, Chemosphere, 2007, 68, 1821–1829 CrossRef CAS PubMed
.
- Y.-F. Guo, D.-Q. Ye, K.-F. Chen, J.-C. He and W.-L. Chen, J. Mol. Catal. A: Chem., 2006, 245, 93–100 CrossRef CAS PubMed
.
- H. Kohno, A. A. Berezin, J.-S. Chang, M. Tamura, T. Yamamoto, A. Shibuya and S. Honda, IEEE Trans. Ind. Appl., 1998, 34, 953–966 CrossRef CAS
.
- J. Li, H. Na, X. Zeng, T. Zhu and Z. Liu, Appl. Surf. Sci., 2014, 311, 690–696 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2015 |




