Facile hydrothermal synthesis of tubular kapok fiber/MnO2 composites and application in supercapacitors†
Weibing Xuab,
Bin Mua,
Wenbo Zhanga and
Aiqin Wang*a
aState Key Laboratory of Solid Lubrication, Center of Eco-Materials and Green Chemistry, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, China. E-mail: aqwang@licp.cas.cn; Fax: +86 931 8277088; Tel: +86 931 4968118
bUniversity of the Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 20th July 2015
Abstract
Kapok fiber/MnO2 (TKF/MnO2) composites with a tubular structure are successfully fabricated via a facile hydrothermal process. Potassium permanganate and kapok fiber served as the manganese source and the template, respectively. The effects of operating parameters including material proportion, reaction temperature, reaction time and the growth mechanism of MnO2 are studied in detail. A maximum specific capacitance of 117 F g−1 has been achieved at 0.25 A g−1 in 1 M Na2SO4 and 95% specific capacitance is maintained after 1000 cycles, which demonstrates the potential application of tubular TKF/MnO2 composites in supercapacitors. The superior electrochemical performances of the obtained composites are attributed to their hollow structure, thin wall thickness, and orderly pore passages, which can facilitate ion diffusion and improve the utilization of the electroactive sites of MnO2.
Introduction
The development of energy storage devices has attracted great attention due to the depletion of fossil fuels and environmental contamination.1–3 Supercapacitors have been considered as a new kind of promising energy storage device with some excellent properties such as high power density, long cycle life, fast charge time, safe operation mode, and capability to bridge the power/energy gap between traditional dielectric capacitors with high power output and batteries/fuel cells with high energy storage.4–15 However, the most intensively explored supercapacitor electrode materials have some inevitable defects related to their structure and performance, which largely limit their wide application in supercapacitors.16–18 For example, the values of capacitance of pure carbon materials, such as activated carbon, carbon black, etc. are limited due to their microstructures, while transition metal oxides, hydroxides and conducting polymers usually possess relative poor cycling stability.19–23An emerging solution to overcome the above drawbacks is to utilize the synergistic effect of binary or ternary hybrid systems composed of conducting polymers, carbon materials and metal oxide. For instance, the hybrid systems composed of polyaniline and metal oxide have been widely investigated to improve the cycle capability of polyaniline and conductivity of metal oxide.24,25 The hybrid material composed of Ni(OH)2 and MnO2 also displayed the enhanced electrochemistry activity.26 In addition, hollow micro-/nano-structured materials have been considered as one of promising electrode materials for supercapacitor due to their unique attractive advantages,27 such as the short diffusion lengths of ions, large material/electrolyte contact area, good strain accommodation, and the unusual mechanical and electrical properties endowed by confining the dimensions.28–30 However, the common synthetic techniques based on hard-template for the preparation of hollow structures are costly and complicated including electrochemical deposition,31 chemical oxidative polymerization,32 electro spinning.33,34
As an agricultural product, kapok fiber (KF) shows a significantly homogeneous hollow tube shape.35–37 It carries large specific surface and good surface activity to makes it much easier to attach metal oxide,38,39 and thus it could be applied in designing the novel electrode materials of supercapacitor. In this paper, the tubular kapok fiber/MnO2 (TKF/MnO2) composites are prepared based on the NaClO2-treated natural KF (TKF) by a simple hydrothermal method in the presence of KMnO4. The hollow structure is kept well during the hydrothermal process, and the possible growth mechanism of MnO2 on the surface of TKF is proposed. The structure–property relationship between the morphology and electrochemical performances of the as-prepared TKF/MnO2 composites are investigated in detail, and the results reveal that the TKF/MnO2 composites shown good electrochemical performance as electrode materials for supercapacitors.
Experiments
Materials
KF is purchased from Shanghai Pan-Da Co., Ltd, China. NaClO2 (chemically pure) is provided by Beijing Hue-Wei Chemical Reagent Co., China. Acetic acid (HAc, Analytical grade) is received from Shanghai Chemical Reagent Factory, Shanghai, China. Potassium permanganate (KMnO4) is analytical reagent grade from Tianjin Chemical Co., China. Ultrapure water (18.25 MΩ cm) is used throughout the experiment.Preparation of tubular TKF/MnO2 composites
KF is firstly pretreated with NaClO2 solution to remove the waxy coatings and to create a hydrophilic surface as described previously.39 The pre-treated Kapok fiber is donated as TKF. Next, the TKF/MnO2 composites are prepared by hydrothermal method in a water system. Taking the synthesis of TKF/MnO2-6 as a representative example, the detailed procedure is presented as follow: 0.4 g TKF is dispersed in 35 mL ultrapure water and stirred for 30 min, and 0.525 g of KMnO4 is added with vigorous magnetic stirring for 5 min. And then the above mixture is sealed in a Teflon-lined stainless steel auto-clave (50 mL capacity) and the temperature is maintained at 140 °C for 1 h. The obtained black TKF/MnO2-6 composite is washed with deionized water and ethanol in sequence after been cooled to room temperature, and then dried in a vacuum at 60 °C for 24 h. The number and preparation conditions of the samples are summarized in Table 1.| Samples | TKF (g) | Time (h) | Temperature (°C) | Capacitancea (F g−1) |
|---|---|---|---|---|
| a The specific capacitance calculated according to eqn (1). | ||||
| TKF/MnO2-1 | 0.2 | 4 | 140 | 3.6 |
| TKF/MnO2-2 | 0.4 | 4 | 140 | 7.4 |
| TKF/MnO2-3 | 0.6 | 4 | 140 | 3.2 |
| TKF/MnO2-4 | 0.8 | 4 | 140 | 2.4 |
| TKF/MnO2-5 | 0.4 | 0.5 | 140 | 42.0 |
| TKF/MnO2-6 | 0.4 | 1 | 140 | 117.0 |
| TKF/MnO2-7 | 0.4 | 2 | 140 | 13.0 |
| TKF/MnO2-8 | 0.4 | 6 | 140 | 9.4 |
| TKF/MnO2-9 | 0.4 | 1 | 100 | 12.5 |
| TKF/MnO2-10 | 0.4 | 1 | 120 | 21.0 |
| TKF/MnO2-11 | 0.4 | 1 | 160 | 33.3 |
Characterization
A Bruker IFS 66 v/s IR spectrometer (Bruker, Karlsruhe, Germany) is used for the FTIR analysis in the range of 400 to 4000 cm−1 with the resolution of 4 cm−1. The morphologies of TKF/MnO2 is characterized using a JEM-1200 EX/S TEM (JEOL, Tokyo, Japan) and an S-4800 field emission SEM (HITACHI, Tokyo, Japan), respectively. TGA is performed on a Perkin Elmer STA6000 thermogravimetric analyzer at a heating rate of 20 °C min−1 under a dry air purge with a flow rate of 200 mL min−1. XRD analysis is conducted by an X-ray powder diffractometer with a Cu anode (PAN analytical Co. X'pert PRO), running at 40 kV and 30 mA. The specific surface areas (SBET) are evaluated by the BET method. The total pore volume (Vtotal) is estimated from the volume of N2 adsorbed at a relative pressure P/P0 = 0.97.Electrochemical analysis
The working electrodes are fabricated with a mixture containing the obtained active materials, carbon black, and polyvinylidenefluoride (PVDF) with the mass ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to make a homogeneous mixture in N,N′-dimethylformamide (DMF).40 Then the slurry is uniformly laid on a foam Ni which is used as a current collector and then dried at 50 °C for 24 h. The foam Ni coated by electroactive material is pressed for 1 min under 10 MPa. All of the measurements are carried out in 1.0 M Na2SO4 electrolyte. The electrochemical behavior of the working electrodes is evaluated by cyclic voltammogram (CV), galvanostatic charge–discharge (GCD) and electrochemical impedance spectroscopy (EIS) using a CHI660E electrochemical working station. All electrochemical experiments are carried out in a three-electrode and two-electrode glass cell, platinum counter electrode, and a standard calomel reference electrode (SCE). CV tests are performed in the potential window ranging from −0.2 to 0.4 V (vs. SCE) at 20, 40, 60, 80 and 100 mV s−1 in 1 M Na2SO4 solution. EIS measurements are carried out in the frequency range from 105 to 0.005 Hz at open circuit potential with a perturbation of 5 mV. The capacitance is calculated from the discharge curves, according to the eqn (1):41
5 to make a homogeneous mixture in N,N′-dimethylformamide (DMF).40 Then the slurry is uniformly laid on a foam Ni which is used as a current collector and then dried at 50 °C for 24 h. The foam Ni coated by electroactive material is pressed for 1 min under 10 MPa. All of the measurements are carried out in 1.0 M Na2SO4 electrolyte. The electrochemical behavior of the working electrodes is evaluated by cyclic voltammogram (CV), galvanostatic charge–discharge (GCD) and electrochemical impedance spectroscopy (EIS) using a CHI660E electrochemical working station. All electrochemical experiments are carried out in a three-electrode and two-electrode glass cell, platinum counter electrode, and a standard calomel reference electrode (SCE). CV tests are performed in the potential window ranging from −0.2 to 0.4 V (vs. SCE) at 20, 40, 60, 80 and 100 mV s−1 in 1 M Na2SO4 solution. EIS measurements are carried out in the frequency range from 105 to 0.005 Hz at open circuit potential with a perturbation of 5 mV. The capacitance is calculated from the discharge curves, according to the eqn (1):41
 | (1) |
Fabrication of TKF/MnO2-6//AC asymmetric supercapacitors
The fabrication of the TKF/MnO2-6//AC asymmetric supercapacitors is conducted by taking the TKF/MnO2-6 binary composites and AC as the positive and negative electrodes, respectively. The 1 M Na2SO4 solution is used as the electrolyte. The masses of the positive and negative electrodes are balanced according to the following eqn (2):41
 | (2) |
 | (3) |
 | (4) |
Result and discussion
The investigation of the effect factors for preparation of TKF/MnO2 composite
The optimum reaction condition is investigated by FTIR, XRD and specific capacitance of the samples prepared at different TKF quality, hydrothermal time, and temperature fixing the addition of KMnO4 (0.525 g) (as showed in Table 1). The specific capacitance can be calculated from GCD curves of the samples according to the eqn (1). The mass ratio of TKF and potassium permanganate is firstly optimized at 140 °C for 4 h. The quality of TKF is selected to be 0.2 g, 0.4 g, 0.6 g and 0.8 g, and the obtained samples are denoted as TKF/MnO2-1, TKF/MnO2-2, TKF/MnO2-3, TKF/MnO2-4, respectively. The FTIR spectra of TKF/MnO2-1–4 are displayed in Fig. S1.† The characteristic peaks appeared at about 1741, 1375 and 1255 cm−1, which could be belonged to the stretching and bending vibrations of the carbonyl bonds of the acetyl ester group in TKF.43 It is clearly seen that the absorption intensity of the three peaks increased significantly with increasing the quality of TKF, which may be assigned to the creasing content of TKF in the corresponding composites. The bands in the 1000–1450 cm−1 region correspond to C–O stretching (ester, or ether of carbohydrate and polysaccharide) and O–H bending vibrations.44 The relative intensity of the peaks is not significantly changed with increasing the quality of TKF. The absorption bands at 517 cm−1 and 620 cm−1 can be attributed to the Mn–O stretching vibration.45 The crystalline structures of as-prepared TKF/MnO2-1–4 are also investigated by XRD, as shown in Fig. S2.† All the diffraction peaks can be indexed to α-MnO2, which are consistent with the reported values (JCPDS Card 44-0141).46 It should be noting that the characteristic peaks of MnO2 become broad and low, which indicates that the crystallinity and the size of the MnO2 particle become poor and small with the increase in the amount of TKF, respectively. That may be due to the content of MnO2 become low in these composites as the amount of TKF increase. The characteristic diffraction peaks of cellulose appear in the position of 2θ close to 22.60° for TKF/MnO2-4 composite, corresponding to the (200) crystallographic planes.47 It is clearly seen that TKF/MnO2-2 composite exhibited better crystalline nature than other TKF/MnO2 composites. It can be seen that the TKF/MnO2-2 composite exhibits the highest specific capacitance, as shown in Table 1. So the most appropriate TKF quality is 0.4 g according to the above analysis.The reaction time is also optimized at 140 °C for different time of 0.5 h, 1 h, 2 h, 4 h, and 6 h, and the obtained samples are denoted as TKF/MnO2-2, 5–8, respectively. The FTIR spectra of TKF/MnO2-2, 5–8 are displayed in Fig. S3.† The characteristic peaks appeared at about 1741, 1375 and 1255 cm−1 decreased significantly with the increasing hydrothermal time from 0.5 h to 1 h. It can be found that three absorption bands are very strong in the TKF/MnO2-5 (0.5 h), but they almost disappear (TKF/MnO2-6) as the hydrothermal time increases to 1 h. Furthermore, the absorption bands in the 1000–1450 cm−1 region also significantly decrease, which may be ascribed to the deacetylation and dehydration occurred during the hydrothermal process.48 It also can be found that the Mn–O stretching vibration is very weak in the TKF/MnO2-5, and the relative intensity of the characteristic absorption bands of Mn–O increases when the reaction time increases to be 1 h, indicating that the content of MnO2 increases with the increasing hydrothermal time. Fig. S4† exhibited the typical XRD patterns of TKF/MnO2-2, 5–8. The diffraction peak of TKF/MnO2-5 is low indicating a poor crystalline feature in these composites. With the increase in the reaction time, the diffraction peaks assigned to α-MnO2 become sharp and high. It indicates that the crystallinity and the size of the MnO2 particle become high and large as the hydrothermal time increase. Therefore, the crystalline and morphology of the MnO2 nanostructures can be controlled very well by the reaction time. The electrochemical performance of the synthesized TKF/MnO2-5–8 composites as electrode materials for supercapacitors is evaluated, as shown in Table 1. It is worth noting that the TKF/MnO2-6 composite exhibits the highest specific capacitance with the value of 117 F g−1. Based on these above, the optimum reaction time is 1 hour in this work.
The reaction temperature is also optimized at different hydrothermal temperature for 1 h with the addition of TKF (0.4 g), as shown in Table 1 of samples of TKF/MnO2-6, 9–11, which corresponded to 100 °C, 120 °C, 140 °C and 160 °C, respectively. As a control, 0.4 g of pure TKF is also hydrothermally treated at 100, 120, 140, and 160 °C for 1 h in order to investigate the structure change of TKF with the increasing hydrothermal temperature. The obtain products are donated as TKF-100, TKF-120, TKF-140, and TKF-160, respectively. The FTIR spectra of the TKF-100–160 are presented in Fig. S5.† By comparing the absorption peaks of four samples, obvious change of absorption peak can be observed. The intensity of absorption peaks at 2914 cm−1 (C–H stretching vibration), 1735 cm−1, 1375 cm−1, and 1254 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ester) decrease with the increasing hydrothermal temperature from 100 °C to 160 °C. Furthermore, the absorption band at 3000–3700 cm−1 becomes broad. This may be ascribed to the deacetylation reaction with an increase of the temperature.48 The digital photos of TKF-100–160 and TKF/MnO2-6, 9–11 are exhibited in Fig. S6.† It clearly indicates that the transformation of the color of the products before and after addition of KMnO4. The FTIR spectra of the TKF/MnO2-6, 9–11 composites are displayed in Fig. S7.† The intensity of the characteristic peaks located at around 1741, 1375 and 1255 cm−1 decreased significantly with the temperature increase from 100 °C to 120 °C. The three peaks almost disappear when the temperature up to 160 °C (TKF/MnO2-11). This result is attributed to the high temperature in favour for the degradation reactions of the acetyl ester group in TKF.49,50 The decreases in the intensity and the broad band at 1000–1460 cm−1 suggest that dehydration occurred with the increasing hydrothermal temperature.44 In addition, the intensity of the characteristic peaks appeared at about 517 cm−1 and 620 cm−1 increase as the temperature elevated from 100 °C to 120 °C. As the temperature further rise, the relative intensity of the two peaks is not significantly change, which may be ascribed to the similar content of the MnO2 in the composite. The XRD patterns of the TKF/MnO2-6, 9–11 composites are displayed in Fig. S8.† The diffraction peak of the cellulous becomes low in the all of composites indicating a poor crystallinity. As for the TKF/MnO2 composites, the diffraction peaks assigned to MnO2 become sharp and high with the increasing hydrothermal temperature, indicating the crystallinity and the size of the MnO2 particle become high and large. However, it should be mentioned that when the reaction temperature up to 160 °C (TKF/MnO2-11), the tubular structure of TKF is destroyed completely (Fig. S9†). This suggests that the TKF is reacted with KMnO4 during hydrothermal process. By contrast, the sample of TKF/MnO2-6 prepared at 140 °C for 1 h exhibits the highest specific capacitance. In summary, the detailed ratio-, time-, and temperature-dependent studies illustrate that the optimum quality of TKF, temperature and reaction time is 0.4 g, 140 °C and 1 h, respectively. Therefore, TKF/MnO2-6 composite is selected as representative for the further study.
O ester) decrease with the increasing hydrothermal temperature from 100 °C to 160 °C. Furthermore, the absorption band at 3000–3700 cm−1 becomes broad. This may be ascribed to the deacetylation reaction with an increase of the temperature.48 The digital photos of TKF-100–160 and TKF/MnO2-6, 9–11 are exhibited in Fig. S6.† It clearly indicates that the transformation of the color of the products before and after addition of KMnO4. The FTIR spectra of the TKF/MnO2-6, 9–11 composites are displayed in Fig. S7.† The intensity of the characteristic peaks located at around 1741, 1375 and 1255 cm−1 decreased significantly with the temperature increase from 100 °C to 120 °C. The three peaks almost disappear when the temperature up to 160 °C (TKF/MnO2-11). This result is attributed to the high temperature in favour for the degradation reactions of the acetyl ester group in TKF.49,50 The decreases in the intensity and the broad band at 1000–1460 cm−1 suggest that dehydration occurred with the increasing hydrothermal temperature.44 In addition, the intensity of the characteristic peaks appeared at about 517 cm−1 and 620 cm−1 increase as the temperature elevated from 100 °C to 120 °C. As the temperature further rise, the relative intensity of the two peaks is not significantly change, which may be ascribed to the similar content of the MnO2 in the composite. The XRD patterns of the TKF/MnO2-6, 9–11 composites are displayed in Fig. S8.† The diffraction peak of the cellulous becomes low in the all of composites indicating a poor crystallinity. As for the TKF/MnO2 composites, the diffraction peaks assigned to MnO2 become sharp and high with the increasing hydrothermal temperature, indicating the crystallinity and the size of the MnO2 particle become high and large. However, it should be mentioned that when the reaction temperature up to 160 °C (TKF/MnO2-11), the tubular structure of TKF is destroyed completely (Fig. S9†). This suggests that the TKF is reacted with KMnO4 during hydrothermal process. By contrast, the sample of TKF/MnO2-6 prepared at 140 °C for 1 h exhibits the highest specific capacitance. In summary, the detailed ratio-, time-, and temperature-dependent studies illustrate that the optimum quality of TKF, temperature and reaction time is 0.4 g, 140 °C and 1 h, respectively. Therefore, TKF/MnO2-6 composite is selected as representative for the further study.
The feasible mechanism
Generally speaking, the synthetic strategies of MnO2-based composites included the redox reaction between Mn2+ and the oxidants (such as MnO4−),51–58 or MnO4− and other reductant (oleic acid, ethylene glycol, or carbon substrate, such as carbon nanotubes, carbon fiber, etc.) to reduce MnO4− to MnO2.59–61 In this study, the loading of manganese dioxide on the surface of TKF are explored by a facile hydrothermal method. The manganese dioxide did not form when the pure potassium permanganate solution are treated at 140 °C for 4 h (Fig. 1a), but the brown MnO2 products are obtained with the addition of the 1 mL of concentrated hydrochloric acid due to the decompose of MnO4− and the redox between MnO4− and Cl− at acidic media according to the reported method.54 What's more, the solution is acidic after hydrothermal treatment completion (Fig. 1b). However, the colour of products is still white when 0.2 g of TKF and 1 mL of the concentrated hydrochloric acid are added into potassium permanganate solution, indicating the manganese dioxide didn't generate in the same hydrothermal conditions after addition of TKF, and the solution is also acidic after hydrothermal treatment completion (Fig. 1c). This phenomenon might be attributed to the dissolving of the generated MnO2 under acidic condition. It is worth noting that the brown TKF/MnO2 composites could be obtained in the presence of TKF after removing the concentrated hydrochloric acid, and the solution is weak alkaline after the reaction completion (Fig. 1d), which also agrees well with the result as depicted in eqn (6). This phenomenon might be attributed to the fact that the hydroxyl groups of the surface of TKF can act as a reductant to react with MnO4− to generate insoluble MnO2. This also might be confirmed from the fabrication of the cellulose/Ag NPs composites used a hydrothermal method without additional reducing reagents.62 The oxidation–reduction potential of silver ions and potassium permanganate at the neutral conditions are shown in the following eqn (5) and (6):| Ag+ + e− → Ag + 0.7996 V | (5) |
| MnO4− + 2H2O + 3e− → MnO2(s) + 4OH− + 0.59 V | (6) |
It is worth noting that the solution is weak alkaline after potassium permanganate decomposed at the neutral conditions according to the eqn (6). Owing to the difference in the reactive activity of the hydroxyl groups between cellulose and TKF, potassium permanganate might be reduced into manganese dioxide using TKF under hydrothermal condition at 140 °C compared with that of cellulose/Ag NPs composites obtained under hydrothermal condition at 120 °C. To our knowledge, the natural fiber/MnO2 composites with tubular structure are prepared for the first time using natural fibers and potassium permanganate as raw materials without added other reductant.
Morphology and structural characterization
The morphologies of the tubular TKF/MnO2 composites and the time-dependent shape evolution of the MnO2 are investigated by SEM, as shown in Fig. 2. At the reaction time 0.5 h, large amount of MnO2 nanoparticles are deposited on the TKF surface as shown in Fig. 2a, and the diameters ranged from 10 nm to 20 nm (Fig. 2b). When the reaction time is increased to 1 h, it can be observed that MnO2 nanoparticles is grown onto the surface of TKF to formed a coaxial structure (Fig. 2c), a lot of irregular shape nanoparticles appeared on the surface of the fibers and the diameters ranged from 40 nm to 50 nm (Fig. 2d). As the reaction proceeded for 2 h, it can be seen that the MnO2 with high density are uniformly distributed on the TKF (Fig. 2e), the irregular shape nanoparticles disappeared and most of the products are nano-tetrahedron with good geometric symmetry. The diameter of nanoparticles further increased up to 80 nm (Fig. 2f). However, upon further increase of the reaction time to 4 h, the diameters of TKF/MnO2 composites is about 450 nm (Fig. 2g), the shape of the loaded MnO2 nanoparticles transformed from tetrahedron to rectangular (Fig. 2h). It is worth noting that the tubular structure of the samples synthesized at different time is maintained very well. It also can be seen that MnO2 nanoparticles confined in the interior of TKF tube only can be observed in the tubing end of TKF, which might greatly increase the capacitance of the composite material. Thereby, this result strongly suggests that the size and morphology of the MnO2 that anchored on the TKF surface can be well-controlled by adjusting the reaction time. | ||
| Fig. 2 SEM images of (a and b) TKF/MnO2-5, (c and d) TKF/MnO2-6, (e and f) TKF/MnO2-7, and (g and h) TKF/MnO2-2. | ||
The energy dispersive X-ray spectroscopy (EDS) analysis of TKF/MnO2-6 demonstrated the distribution of manganese, oxygen, carbon components of TKF/MnO2-6 (Fig. 3a–d). It is obvious that the above three components are uniformly distributed on the tubular TKF/MnO2-6 composites. This result implied that the MnO2 nanoparticles are fully confined within the surface of tubular TKF during the hydrothermal treatment, and the two components (TKF, MnO2) are strongly coupled together in the hybrids. In addition, the samples which prepared at 0.5 h (TKF/MnO2-5) and 2 h (TKF/MnO2-7) are also characterized by EDS (ESI Fig. S10 and S11†), and the manganese content of the two samples is 23.4% and 29.2%, respectively. It is higher than the samples of TKF/MnO2-6 (20.83%). According to the hydrothermal process of the pure carbohydrates.63 This process mainly includes three important steps: (1) dehydration of the carbohydrate to (hydroxymethyl) furfural; (2) polymerization towards polyfurans; (3) carbonization via further intermolecular dehydration. The samples of 5–7 are prepared at the hydrothermal temperature 140 °C for 0.5 h, 1 h, and 2 h, respectively. Only the first step of dehydration might take place during this process accompanied with the release of some gases, such as CO2. The ratio of C/O of the samples of 5–7 is 1.584, 1.384, and 1.523 with the increase in the reaction time. The changes in the manganese content of samples might be related to the ratio of C/O during the hydrothermal process. Furthermore, the value of capacitance of TKF/MnO2-5 and TKF/MnO2-7 is less than that of TKF/MnO2-6 (Table 1), and this result strongly suggests that the size and morphology of the MnO2 anchored on the TKF surface has a great impact on specific capacitance of composite.
 | ||
| Fig. 3 (a–d) Element mapping images of TKF/MnO2-6 (e) EDX curve of TKF/MnO2-6, the inset shows the atomic ratio of Mn, O and C. | ||
In order to further comparison the structure change of the KF, TKF, TKF-140 and TKF/MnO2 composites, the FTIR spectra of the pristine KF, TKF, TKF-140 and the TKF/MnO2-6 composite are showed in Fig. 4. It can be seen the broad absorption band at about 3393 cm−1 characteristic of the stretching vibration of –OH in cellulose of KF. The absorption band at 2914 cm−1 is assigned to asymmetric and symmetric stretching vibration in –CH2 and –CH3 group in the acetyl ester groups of cellulose in the KF. The three important ester bands at 1741, 1375 and 1255 cm−1 are associated with the stretching and bending vibrations of carbonyl bonds (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of the acetyl ester group in the KF. The stretching vibration of C–C in different substituted aromatic rings in lignin at 1592 and 1463 cm−1 in KF. The bands in the 1000–1450 cm−1 region correspond to C–O stretching (ester, or ether of carbohydrate and polysaccharide) and O–H bending vibrations. When the KF is treated with NaClO2, the absorption band of the hydroxyl groups becomes broad. Furthermore, the relative intensity of the absorption peaks at 2914 cm−1, 1735 cm−1, 1375 cm−1, 1254 cm−1, and 1000–1450 cm−1 increase. It is reported that the NaClO2 would produce chlorine dioxide under an acidic condition, which rendered the oxidation of lignin.64 Due to part of hydrogen bonds and lignin is broken, thus leading to increase in the amorphous part in cellulose and release of more hydroxyl groups. The absorption bands at 1592 and 1463 cm−1 almost disappear in TKF, which indicates the broken of the lignin in the TKF. The TKF exhibited good hydrophilic property and it is fully wet after stirred with distilled water for 30 min. When the TKF is processed at 140 °C for 1 hour without addition KMnO4, the relative intensity of the absorption peaks at 2914 cm−1, 1735 cm−1, 1375 cm−1, 1254 cm−1, and 1000–1450 cm−1 further increase, which may be ascribed to dehydration occurred during the hydrothermal process.44 However, the characteristic peaks appeared at about 1741, 1375 and 1255 cm−1 almost disappears, as shown in the FTIR spectrum of TKF/MnO2-6 composite. This result may be due to the deacetylation by the cleavage of acetyl groups linked as an ester group to the celluloses in TKF.48–50 Furthermore, the weak alkaline condition will be help to the deacetylation reaction, which result from the reduction reaction of KMnO4 under neutral conditions as depicted in eqn (6). This result also agrees well with that of Fig. 1d. The stretching vibration of –OH in cellulose undergoes a shift from 3393 cm−1 in the TKF to 3430 cm−1 in the TKF/MnO2-6 composite. In addition, the relative intensity of absorption band decrease significantly accompanied with a red shift in the 1000–1450 cm−1 region. This phenomenon may be associated to the anchor of MnO2 particle on the TKF surface. The characteristic peaks of MnO2 appear at about 628 and 508 cm−1, which could belonged to the Mn–O vibrations of MnO6 octahedral in α-MnO2 (Fig. 4). The peak located at 1648 cm−1 could be related to the bending vibration mode of the absorbed water.65
O) of the acetyl ester group in the KF. The stretching vibration of C–C in different substituted aromatic rings in lignin at 1592 and 1463 cm−1 in KF. The bands in the 1000–1450 cm−1 region correspond to C–O stretching (ester, or ether of carbohydrate and polysaccharide) and O–H bending vibrations. When the KF is treated with NaClO2, the absorption band of the hydroxyl groups becomes broad. Furthermore, the relative intensity of the absorption peaks at 2914 cm−1, 1735 cm−1, 1375 cm−1, 1254 cm−1, and 1000–1450 cm−1 increase. It is reported that the NaClO2 would produce chlorine dioxide under an acidic condition, which rendered the oxidation of lignin.64 Due to part of hydrogen bonds and lignin is broken, thus leading to increase in the amorphous part in cellulose and release of more hydroxyl groups. The absorption bands at 1592 and 1463 cm−1 almost disappear in TKF, which indicates the broken of the lignin in the TKF. The TKF exhibited good hydrophilic property and it is fully wet after stirred with distilled water for 30 min. When the TKF is processed at 140 °C for 1 hour without addition KMnO4, the relative intensity of the absorption peaks at 2914 cm−1, 1735 cm−1, 1375 cm−1, 1254 cm−1, and 1000–1450 cm−1 further increase, which may be ascribed to dehydration occurred during the hydrothermal process.44 However, the characteristic peaks appeared at about 1741, 1375 and 1255 cm−1 almost disappears, as shown in the FTIR spectrum of TKF/MnO2-6 composite. This result may be due to the deacetylation by the cleavage of acetyl groups linked as an ester group to the celluloses in TKF.48–50 Furthermore, the weak alkaline condition will be help to the deacetylation reaction, which result from the reduction reaction of KMnO4 under neutral conditions as depicted in eqn (6). This result also agrees well with that of Fig. 1d. The stretching vibration of –OH in cellulose undergoes a shift from 3393 cm−1 in the TKF to 3430 cm−1 in the TKF/MnO2-6 composite. In addition, the relative intensity of absorption band decrease significantly accompanied with a red shift in the 1000–1450 cm−1 region. This phenomenon may be associated to the anchor of MnO2 particle on the TKF surface. The characteristic peaks of MnO2 appear at about 628 and 508 cm−1, which could belonged to the Mn–O vibrations of MnO6 octahedral in α-MnO2 (Fig. 4). The peak located at 1648 cm−1 could be related to the bending vibration mode of the absorbed water.65
The phase purity and crystallographic structure of the TKF/MnO2-6 have been identified by X-ray power diffraction. The XRD patterns of the TKF/MnO2-6 composites and the TKF are shown in Fig. 5. It can be found that the characteristic diffraction peaks of cellulose appear in the position of 2θ close to 15.59° and 22.60° for TKF, corresponding to the (110) and (200) crystallographic planes. In case of TKF/MnO2-6 composite, the characteristic diffraction peaks of cellulose almost disappear. This may be due to the crystallinity has been destroyed during the hydrothermal process. All the diffraction peaks can be indexed to α-MnO2, which are consistent with the reported values (JCPDS Card 44-0141). No characteristic impurity peaks are observed, indicating the high purity of the sample.
The TGA curves of TKF and the TKF/MnO2-6 composite are showed in Fig. 6. The TKF showed a dramatic mass loss (about 78% of weight) from around 250 °C to 350 °C, due to the decomposition of oxygen-containing groups.51 The 45% weight loss accompanied by an endothermic reaction is observed at 140–450 °C at the curve of the TKF/MnO2-6 composite assigned to the loss of water produced by the decomposition and dehydroxylation of the TKF and the high valence MnOx decomposed to a lower valence state along with the removal of more stable oxygen.52,53 As calculated from the TGA curves, the MnO2 content of TKF/MnO2-6 composite is about 23 wt%.
The detailed pore textural characteristics of the samples are analyzed by nitrogen adsorption–desorption technique. The BET specific surface areas and pore volumes of TKF and TKF/MnO2 composites are summarized in Table S1.† It can be found that the TKF exhibits the smallest surface area and pore volume with 0.8875 m2 g−1 and 0.0012 cm3 g−1, respectively. This result is attributed to the TKF presents a regular hollow tubular structure with outer and inner diameters of 20–25 and 16–23 μm, and the BET technique cannot detect the surface areas and pore volumes.66 When the MnO2 is anchored on the surface of TKF, the specific surface areas and pore volumes of all TKF/MnO2 composites can be detected due to the introduction of MnO2 nanoparticles. It should be noting that the surface areas and pore volumes of the TKF/MnO2 composites increase significantly with the increase in the hydrothermal time from 0.5 h to 1 h. At the hydrothermal time 1 h, the TKF/MnO2-6 composite exhibits the maximum the surface areas and pore volumes up to 222.41 m2 g−1 and 0.496 cm3 g−1. As the hydrothermal time further increase (TKF/MnO2-2, 7–8), the surface areas and pore volumes greatly decreased. It might be assigned to the change of the MnO2 morphology with increase in the hydrothermal time, as depicted in Fig. 2. The diameters of the manganese dioxide particles anchored on the surface of TKF become larger after being hydrothermally treated for 1 h, which might be result in the decrease in the surface areas and pore volumes. It also can be observed that the surface area and pore volume also are affected by the hydrothermal temperature. The BET specific surface areas and pore volumes increased with the increasing hydrothermal temperature and the pore structure parameters reach the maximum value at 140 °C (TKF/MnO2-6). This may be ascribed to the fact that the high temperature is favor for the formation of manganese dioxide. However, the pore structure parameters of TKF/MnO2-11 decrease while the hydrothermal temperature is up to 160 °C. This decrease might be related to the collapse of the hollow structure of composites as shown in the SEM images (Fig. S9†).
The Fig. 7 shows the nitrogen adsorption–desorption isotherms and pore size distributions of TKF and TKF/MnO2-6 composite. For TKF/MnO2-6 sample, the nitrogen adsorption isotherms show an evident type IV isotherm, and a type H4 hysteresis loop also can be observed in the adsorption–desorption isotherms (Fig. 7a), indicating the existence of mesopore size in the composite.67,68 The slope shape increase at the medium relative pressure of 0.35–0.65 P/P0 associated with capillary condensation taking place in mesopores, illustrating the presence of well developed mesoporosity.69,70 Fig. 7b shows the pore size distribution of TKF/MnO2-6. It can be seen that the porosity of the composite is essentially consisted of mesopores, in which the average pore size is around 8.92 nm. In the case of the TKF, there is no significant adsorption–desorption occurs during the increase pressure process, this result is ascribed to the fact that the diameter of the hollow tubular structure of TKF is up to micron level, and the pore size distribution of the TKF is not detected. In addition, the nitrogen adsorption–desorption isotherms and pore size distributions of TKF/MnO2-2, 5–11 composites are tested as shown in Fig. S12.† The adsorption–desorption isotherms of all these samples are exhibit a typical IV isotherm with a H3 hysteresis loop. It worth noting that mesopores are dominated in the all samples and the pore size is around range from 5.12 nm to 30 nm. For comparison, it is clearly found that surface areas and the pore volumes of TKF/MnO2-6 are the largest among all of the samples.
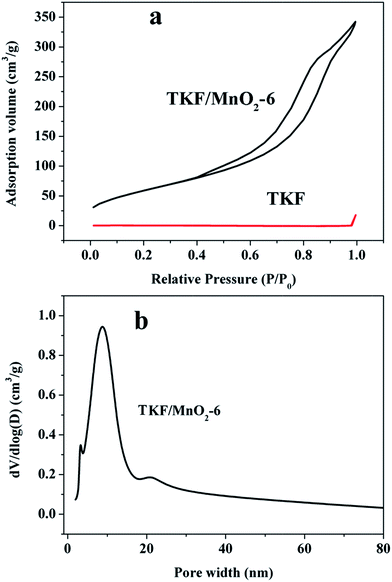 | ||
| Fig. 7 (a) N2 adsorption–desorption isotherms of TKF and TKF/MnO2-6 composite and (b) BJH pore size distribution of TKF/MnO2-6 composites. | ||
Electrochemical properties
To explore the potential applications of the TKF/MnO2-6 composite, the sample is fabricated into supercapacitor electrode for the electrochemical measurement. Fig. 8a showed the typical CV curves of the TKF/MnO2-6 at different scanning rates, which indicates that the electrode materials retain good electro-chemical properties and quickly undergo ion exchange in the charge–discharge process. However, the effective interaction between the ions and the electrode decreased greatly with the increasing of the scanning rate. Meanwhile, these curves displayed no peaks in the range between −0.2 and 0.4 V, which demonstrated that the composite electrodes are charged and discharged at a pseudo constant rate over the complete voltammetric cycle.71 Fig. 8b showed the GCD curves of the TKF/MnO2-6 composite electrodes at different current densities. The specific capacitance of TKF/MnO2-6 composite electrodes decreased from 117 F g−1 to 95 F g−1 with the increase in the current density from 0.25 A g−1 to 4 A g−1. It could be calculated that about 80% of the capacitance of the composite electrode is retained.To obtain more information about the ability of the TKF/MnO2-6 composite to function as electrodes in supercapacitors, EIS measurements are carried out in 1.0 M Na2SO4 solution at open circuit potential (vs. SCE), which could provide useful information about the redox reaction resistance and equivalent series resistance. Fig. 8c presents the typical Nyquist plots of the composite. At the high-frequency region, the real axis provides quantitative information on the effective internal resistance (Rs), which is mainly attributed to the uncompensated solution resistance. It can be seen that the Rs values of TKF/MnO2-6 is about 2.0 Ω. The semicircle corresponded to the charge-transfer resistance (Rct) at the electrode–electrolyte interface. It could be seen that the diameter of the capacitive reactance arc of the TKF/MnO2-6 electrode is about 5.0 Ω. It could be inferred that the charge transfer resistance of electrode materials prepared with TKF/MnO2-6 composites is small, and it facilitates charge-transfer and the migration of the electrolyte ions in the interior of the pores of electrode materials. Furthermore, the line at low frequencies is closer to a parallel line along the imaginary axis for an ideal capacitor.
To evaluate the durability of the TKF/MnO2-6 composite, the long-term cycling stability of the TKF/MnO2-6 electrodes is also tested by continuous cyclic voltammetric scans at the scan rates of 100 mV s−1 for 1000 cycles in 1 M Na2SO4 solution and the result is shown in Fig. 8d. The capacitance retention rate can maintain 95% of its original specific capacitance after 1000 cycles, which could be attributed to the introduction of TKF, and hollow tubular structure of the as-prepared composites. The hollow channels can not only promote accessibility of electrolyte ions to active sites and fast diffusion, but also shorten the transport length for ions and storage charge during charging/discharging processes effectively.72
An asymmetric supercapacitor is fabricated based on the TKF/MnO2-6 composites to further value its application. More specifically, the TKF/MnO2-6 composite is used as the positive electrode and the commercial activated carbon (AC) is treated as the negative electrode, and the mass ratio of positive and negative is about 1.4 according to the eqn (2). The electrochemistry test results of the asymmetric supercapacitor are shown in Fig. 8. Based on the three-electrode electrochemical measurements of both TKF/MnO2-6 composite and AC electrode, a certain mass of AC supported on Ni foam is chosen to balance the capacitance of two electrodes.73 The CV curves of the asymmetric capacitor shows that the cell voltage could be extended to 2.0 V (Fig. 9a). The typical CV result at various scan rates indicates ideal capacitor behaviour, with a nearly rectangular CV shape. The GCD curves (Fig. 9b) exhibited great charge–discharge potentials that are nearly proportional to the charge or discharge time. The specific capacitances calculated from the GCD curves at current density 0.25 A g−1 is 50 F g−1. The energy density is 27.8 W h kg−1 and the power density is 330 W kg−1 according to the eqn (3) and (4). The results indicates that the supercapacitor made by our method have a great superiority in the shortage of energy density. Moreover, EIS is applied in this study in order to understand the interfacial process of the asymmetric supercapacitor. The data is presented in Fig. 9c in the Nyquist plot form. The semicircle and the oblique line indicate that the electrochemical reaction is controlled by charge transfer at high frequency range and electrolyte diffusion at low frequency range.
 | ||
| Fig. 9 (a) CV curves at different scan rates, (b) GCD curves at different current density, and (c) EIS plots in 1 M Na2SO4 electrolyte for the TKF/MnO2-6//AC supercapacitor. | ||
Table S2† demonstrates the comparison in the capacitive performance of supercapacitors based on various natural fiber composites presented in literature and this work. It can be seen that the capacitive performance of the present TKF/MnO2 composites surpasses many other natural fiber composites. In addition, the TKF/MnO2 composites facilely fabricated through a green hydrothermal process compared with the reported complicated preparation process of other natural fiber composites. Furthermore, in comparison with the high price of other supports, such as carbon nanotube, graphene gel, the present TKF is quite cost-effective and it can be produced on large-scale.
Conclusions
A series of TKF/MnO2 composites have been prepared successfully by the hydrothermal method. The results of electrochemical analysis showed that the specific capacitance of the TKF/MnO2-6 composites prepared at 140 °C for 1 h with the addition of 0.4 g TKF is 117 F g−1 at 0.25 A g−1 in 1.0 M Na2SO4 solution. Furthermore, the composite displayed good cycle stability and retained 95% of its original specific capacitance after 1000 cycles by consecutive cyclic voltammetric scans at the scan rate of 100 mV s−1. An asymmetric supercapacitor is further fabricated based on the TKF/MnO2-6 positive electrode with corresponding AC as negative electrode. The TKF/MnO2-6//AC asymmetric supercapacitor obtained the highest energy density of 27.8 W h kg−1 with mass ratio of 1.4 at charge–discharge current density of 0.25 A g−1, with the corresponding power density of 330 W kg−1. Therefore, the obtained composites are expected to be promising electrode materials for designing high-performance supercapacitors.Acknowledgements
The authors gratefully acknowledge the support of the National Natural Science Foundation of China (No. 51303190).Notes and references
- W. Chen, C. Xia and H. N. Alshareef, ACS Nano, 2014, 8, 9531–9541 CrossRef CAS PubMed.
- J. Zhu, Z. Xu and B. G. Lu, Nano Energy, 2014, 7, 114–123 CrossRef CAS PubMed.
- Z. Y. Wang and C. J. Liu, Nano Energy, 2015, 11, 277–293 CrossRef CAS PubMed.
- J. Zhu, G. H. Zhang, X. Z. Yu, Q. H. Li and B. G. Lu, Nano Energy, 2014, 3, 80–87 CrossRef CAS PubMed.
- Y. Z. Su, S. Li, D. Q. Wu, F. Zhang, H. W. Liang, P. F. Gao, C. Cheng and X. L. Feng, ACS Nano, 2012, 6, 8349–8356 CrossRef CAS PubMed.
- X. L. Ma, G. Q. Ning, C. L. Qi, C. G. Xu and J. S. Gao, ACS Appl. Mater. Interfaces, 2014, 6, 14415–14422 CAS.
- F. W. Yuan and H. Y. Tuan, Chem. Mater., 2014, 26, 2172–2179 CrossRef CAS.
- X. J. Hou, X. F. Wang, B. Liu, Q. F. Wang, T. Luo, D. Chen and G. Z. Shen, Nanoscale, 2014, 6, 8858–8864 RSC.
- H. Long, T. L. Shi, H. Hu, S. L. Jiang, S. Xi and Z. R. Tang, Sci. Rep., 2014, 4, 1–9 Search PubMed.
- J. P. Liu, J. Jiang, C. W. Cheng, H. X. Li, J. X. Zhang, H. Gong and H. J. Fan, Adv. Mater., 2011, 23, 2076–2081 CrossRef CAS PubMed.
- X. Z. Yu, B. G. Lu and Z. Xu, Adv. Mater., 2014, 26, 1044–1051 CrossRef CAS PubMed.
- H. S. Choi and C. R. Park, J. Power Sources, 2014, 259, 1–14 CrossRef CAS PubMed.
- W. F. Wei, X. W. Cui, W. X. Chen and D. G. Ivey, Chem. Soc. Rev., 2011, 40, 1697–1721 RSC.
- L. Y. Yuan, X. H. Lu, X. Xiao, T. Zhai, J. J. Dai, F. C. Zhang, B. Hu, X. Wang, L. Gong, J. Chen, C. G. Hu, Y. X. Tong, J. Zhou and Z. L. Wang, ACS Nano, 2011, 6, 656–661 CrossRef PubMed.
- H. C. Chen, J. J. Jiang, L. Zhang, H. Z. Wan, T. Qi and D. D. Xia, Nanoscale, 2013, 5, 8879–8883 RSC.
- J. Jiang, Y. Y. Li, J. P. Liu, X. T. Huang, C. Z. Yuan and X. W. Lou, Adv. Mater., 2012, 24, 5166–5180 CrossRef CAS PubMed.
- M. K. Liu, W. W. Tjiu, J. S. Pan, C. Zhang, W. Gao and T. X. Liu, Nanoscale, 2014, 6, 4233–4242 RSC.
- G. A. Snooka, P. Kaob and A. S. Bestb, J. Power Sources, 2011, 196, 1–12 CrossRef PubMed.
- C. G. Liu, Z. N. Yu, D. Neff, A. Zhamu and B. Jang, Nano Lett., 2010, 10, 4863–4868 CrossRef CAS PubMed.
- E. Frackowiak, Phys. Chem. Chem. Phys., 2007, 9, 1774–1785 RSC.
- D. N. Futaba, K. Hata, T. Yamada, T. Hiraoka, Y. Hayamizu, Y. Kakudate, O. Tanaike, H. Hatori, M. Yumura and S. Iijima, Nat. Mater., 2006, 5, 987–994 CrossRef CAS PubMed.
- X. Y. Lang, A. Hirata, T. Fujita and M. W. Chen, Nat. Nanotechnol., 2011, 6, 232–236 CrossRef CAS PubMed.
- J. Lee, J. Kim and T. Hyeon, Adv. Mater., 2006, 18, 2073–2094 CrossRef CAS PubMed.
- J. Han, L. Y. Li, P. Fang and R. Guo, J. Phys. Chem. C, 2012, 116, 15900–15907 CAS.
- S. P. Yu, X. C. Chang, Z. M. Wang, K. F. Han and H. Zhu, Adv. Mater. Res., 2011, 239, 2042–2045 CrossRef.
- H. Jiang, C. Z. Li, T. Sun and J. Ma, Chem. Commun., 2012, 48, 2606–2608 RSC.
- C. Guan, X. H. Xia, N. Meng, Z. Y. Zeng, X. H. Cao, C. Soci, H. Zhang and H. J. Fan, Energy Environ. Sci., 2012, 5, 9085–9090 CAS.
- X. Y. Lai, J. E. Halpert and D. Wang, Energy Environ. Sci., 2012, 5, 5604–5618 CAS.
- J. Y. Wang, N. L. Yang, H. J. Tang, Z. H. Dong, Q. Jin, M. Yang, D. Kisailus, H. J. Zhao, Z. Y. Tang and D. Wang, Angew. Chem., 2013, 125, 6545–6548 CrossRef PubMed.
- Y. E. Miao, W. Fan, D. Chen and T. X. Liu, ACS Appl. Mater. Interfaces, 2013, 5, 4423–4428 CAS.
- Z. L. Wang, R. Guo, G. R. Li, H. L. Lu, Z. Q. Liu, F. M. Xiao, M. Q. Zhang and Y. X. Tong, J. Mater. Chem., 2012, 22, 2401–2404 RSC.
- Z. B. Lei, Z. W. Chen and X. S. Zhao, J. Phys. Chem. C, 2010, 114, 19867–19874 CAS.
- B. Ren, M. Q. Fan, Q. Liu, J. Wang, D. L. Song and X. F. Bai, Electrochim. Acta, 2013, 92, 197–204 CrossRef CAS PubMed.
- X. Wu, Y. Zeng, H. R. Gao, J. Su, J. P. Liu and Z. H. Zhu, J. Mater. Chem. A, 2013, 1, 469–472 CAS.
- Y. A. Zheng, W. B. Wang, D. J. Huang and A. Q. Wang, Chem. Eng. J., 2012, 191, 154–161 CrossRef CAS PubMed.
- W. B. Wang, F. F. Wang, Y. R. Kang and A. Q. Wang, Chem. Eng. J., 2014, 237, 336–343 CrossRef CAS PubMed.
- T. T. Lim and X. F. Huang, Chemosphere, 2007, 66, 955–963 CrossRef CAS PubMed.
- K. Hori, M. E. Flavier, S. Kuga, T. B. T. Lam and K. Iiyama, J. Wood Sci., 2000, 46, 401–404 CrossRef CAS.
- K. J. Hwang, C. H. Hwang, I. H. Lee, T. Kim, S. Jin and J. Y. Park, Biomass Bioenergy, 2014, 68, 62–66 CrossRef CAS PubMed.
- W. Tang, W. Li, X. Shan, X. Wu and Y. Chen, Mater. Lett., 2015, 140, 95–98 CrossRef CAS PubMed.
- S. K. Meher, P. Justin and G. Ranga Rao, ACS Appl. Mater. Interfaces, 2011, 3, 2063–2073 CAS.
- A. K. Singh, D. Sarkar, G. G. Khan and K. Mandal, J. Mater. Chem. A, 2013, 1, 12759–12767 CAS.
- Y. A. Zheng, J. T. Wang, Y. F. Zhu and A. Q. Wang, J. Environ. Sci., 2015, 27, 21–32 CrossRef PubMed.
- M. Sevilla and A. B. Fuertes, Chem.–Eur. J., 2009, 15, 4195–4203 CrossRef CAS PubMed.
- P. Ragupathy, D. H. Park, G. Campet, H. N. Vasan, S. J. Hwang, J. H. Choy and N. Munichandraiah, J. Phys. Chem. C, 2009, 113, 6303–6309 CAS.
- Y. K. Hsu, Y. C. Chen, Y. G. Lin, L. C. Chen and K. H. Chen, Chem. Commun., 2011, 47, 1252–1254 RSC.
- B. Mu, Y. A. Zheng and A. Q. Wang, Cellulose, 2015, 22, 615–624 CrossRef CAS.
- B. F. Tjeerdsma and H. Militz, Holz Roh- Werkst., 2005, 63, 102–111 CrossRef CAS.
- F. Carrasco and C. Roy, Wood Sci. Technol., 1992, 26, 189–208 CAS.
- R. M. Rowell, A. M. Tillman and R. Simonson, J. Wood Chem. Technol., 1986, 6, 427–448 CrossRef CAS PubMed.
- S. Chen, J. W. Zhu, X. D. Wu, Q. F. Han and X. Wang, ACS Nano, 2010, 4, 2822–2830 CrossRef CAS PubMed.
- Y. Qian, S. B. Lu and F. L. Gao, J. Mater. Sci., 2011, 46, 3517–3522 CrossRef CAS.
- S. Devaraj and N. Munichandraiah, J. Phys. Chem. C, 2008, 112, 4406–4417 CAS.
- S. J. He and W. Chen, J. Power Sources, 2014, 262, 391–400 CrossRef CAS PubMed.
- P. Wang, Y. J. Zhao, L. X. Wen, J. F. Chen and Z. G. Lei, Ind. Eng. Chem. Res., 2014, 53, 20116–20123 CrossRef CAS.
- M. Huang, X. L. Zhao, F. Li, L. L. Zhang and Y. X. Zhang, J. Power Sources, 2015, 277, 36–43 CrossRef CAS PubMed.
- V. Subramanian, H. W. Zhu, R. Vajtai, P. M. Ajayan and B. Q. Wei, J. Phys. Chem. B, 2005, 109, 20207–20214 CrossRef CAS PubMed.
- G. H. Yu, L. B. Hu, M. Vosgueritchian, H. L. Wang, X. R. Xie, J. McDonough, X. Cui, Y. Cui and Z. N. Bao, Nano Lett., 2011, 11, 2905–2911 CrossRef CAS PubMed.
- M. W. Xu, L. B. Kong, W. J. Zhou and H. L. Li, J. Phys. Chem. C, 2007, 111, 19141–19147 CAS.
- B. Mu, W. B. Zhang, S. J. Shao and A. Q. Wang, Phys. Chem. Chem. Phys., 2014, 16, 7872–7880 RSC.
- L. Zhou, J. H. He, J. Zhang, Z. C. He, Y. C. Hu, C. B. Zhang and H. He, J. Phys. Chem. C, 2011, 115, 16873–16878 CAS.
- J. J. Wu, N. Zhao, X. L. Zhang and J. Xu, Cellulose, 2012, 19, 1239–1249 CrossRef CAS.
- N. Baccile, M. Antonietti and M. Titirici, ChemSusChem, 2010, 3, 246–253 CrossRef CAS PubMed.
- J. T. Wang, Y. A. Zheng and A. Q. Wang, Mar. Pollut. Bull., 2013, 69, 91–96 CrossRef CAS PubMed.
- Y. Liu, J. T. Wang, Y. A. Zheng and A. Q. Wang, Chem. Eng. J., 2012, 184, 248–255 CrossRef CAS PubMed.
- M. Eddaoudi, J. Am. Chem. Soc., 2005, 127, 14117 CrossRef CAS.
- Y. Lei, J. Li, Y. Y. Wang, L. Gu, Y. F. Chang, H. Y. Yuan and D. Xiao, ACS Appl. Mater. Interfaces, 2014, 6, 1773–1780 CAS.
- S. M. Kang, X. L. Li, J. Fan and J. Chang, Ind. Eng. Chem. Res., 2012, 51, 9023–9031 CrossRef CAS.
- M. D. Chen, X. Y. Kang, T. Wumaier, J. Q. Dou, B. Gao, Y. Han, G. Q. Xu, Z. Q. Liu and L. Zhang, J. Solid State Electrochem., 2013, 17, 1005–1012 CrossRef CAS.
- L. Zhou, H. Cao, S. Q. Zhu, L. R. Hou and C. Z. Yuan, Green Chem., 2015, 17, 2373–2382 RSC.
- T. Y. Wei, X. L. Wei, Y. Gao and H. M. Li, Electrochim. Acta, 2015, 169, 186–194 CrossRef CAS PubMed.
- J. P. Du, R. H. Zhao and G. R. Jiao, Int. J. Hydrogen Energy, 2013, 38, 5789–5795 CrossRef CAS PubMed.
- X. H. Tang, H. J. Li, Z. H. Liu, Z. P. Yang and Z. L. Wang, J. Power Sources, 2011, 196, 855–859 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: FTIR spectra and XRD patterns of TKF/MnO2-1–11, SEM of TKF/MnO2-11, FTIR spectra and digital photos of TKF-100–160 and TKF/MnO2-6, 9–11, EDS of TKF/MnO2-5 and TKF/MnO2-7, the BET specific surface areas and volumes of TKF and TKF/MnO2-2, 5–11 composites are summarized in Table S1, the nitrogen adsorption–desorption isotherms and pore size distributions of TKF and TKF/MnO2-2, 5–11 composites, CV, GCD and ESI analysis of TKF/MnO2-2, 5–11, the comparison in the capacitive performance of supercapacitors based on various natural fiber composites. See DOI: 10.1039/c5ra13602d |
| This journal is © The Royal Society of Chemistry 2015 |





