pH-modulated double LCST behaviors with diverse aggregation processes of random-copolymer grafted silica nanoparticles in aqueous solution†
Abstract
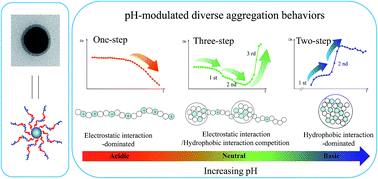
Thermo-responsive hybrid nanoparticles composed of silica-core and poly(N,N-dimethylaminoethyl methacrylate-co-N-isopropylacrylamide) P(DMAEMA-co-NIPAM) copolymer-shell were prepared through a one-pot surface-initiated atom transfer radical polymerization (ATRP) technique. The well-defined core–shell hybrid nanoparticles with copolymer shell of uniform thickness were revealed by transmission electron microscopy (TEM). The thermo-responsive behavior of the hybrid nanoparticles in aqueous solutions was evaluated through combined techniques, including ultraviolet-visible spectrophotometer (UV-vis) and dynamic light scattering (DLS) analysis. Interestingly, pH-modulated LCST behavior with diverse aggregation processes can be observed. Specifically, the random copolymer grafted nanoparticles presented an LCST behavior with one-step transition process of shrink in acidic solution, a double LCST behavior with three-step transition process of shrink–shrink–aggregation in neutral solution, and a double LCST behavior with two-step transition process of assembly-shrink/aggregation in basic solution. The difference was attributed to the pH-modulated imbalances of electrostatic repulsion and hydrophobic interaction of the attached copolymer chains. Overall, these results disclose that pH and temperature can act as efficient modulators for the programmable control and fine-tuning of the morphology and aggregate size of the core–shell functionalized nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...