Potential role of protein stabilizers in amelioration of Parkinson's disease and associated effects in transgenic Caenorhabditis elegans model expressing alpha-synuclein
Supinder Kaurab and
Aamir Nazir*ab
aAcademy of Scientific and Innovative Research (AcSIR), Anusandhan Bhawan, New Delhi, India
bLaboratory of Functional Genomics and Molecular Toxicology, Division of Toxicology, CSIR-Central Drug Research Institute, Lucknow 226031, India. E-mail: anazir@cdri.res.in; Fax: +91-522-2623405; Tel: +91-522-2612411
First published on 8th September 2015
Abstract
Protein stabilizers/chemical chaperones/osmolytes find significant industrial application in maintaining the native state of proteins and averting their misfolding or aggregation during repetitive freeze-thawing and various other stresses. Existing literature presents evidence towards possible therapeutic benefits of these protein stabilizers in evading or delaying disorders arising from protein misfolding and aggregation, usually termed as proteo-pathies. Parkinson's disease (PD) is one such disorder marked with alpha synuclein aggregation forming inclusions resulting in dopaminergic neurodegeneration and impaired motor function. We carried out this study in order to delineate the potential of protein stabilizers in attenuating manifestations associated with parkinsonism using a transgenic Caenorhabditis elegans model expressing human alpha synuclein. We studied protein stabilizers sorbitol, trehalose and xylitol and evaluated various endpoints associated with alpha synuclein aggregation. Lower concentrations of protein stabilizers were inefficient in exhibiting any effect, however a higher concentration (10 mM) did exhibit a significant effect on the studied phenotypes. Trehalose, at 10 mM concentration, showed reduction in alpha synuclein levels and reactive oxygen species, while showing significant increase in motility, dopamine levels and up-regulation of autophagic and chaperonic genes bec-1, lgg-1, epg-8, hsp-60 and hsp-4 in alpha synuclein expressing model, implicating its beneficial effects in parkinsonism. Our studies established the potential role of trehalose in alleviating manifestations associated with parkinsonism via its inherent activity and through induction of autophagic machinery in vivo. We conclude that these chemical chaperones have potential in being used as a supplementary therapeutic intervention addressing the important factor of protein aggregation in neurodegenerative Parkinson's disease. Further research on the subject will be required towards establishing their mechanism of action and suitability of such intervention, possibly along with existing therapies that address other important factors of the disease.
Introduction
Protein stabilizers or osmolytes are a class of simple, biocompatible compounds bearing potential to alleviate protein aggregation and correct misfolded proteins. With the mechanism of action still unknown1–3 there are studies implicating the action of these molecules on different parts of the protein quality control system, inducing expression of various molecular chaperones.4,5 Molecular chaperones, amino acids and osmolytes can potentiate rescue of aggregated proteins, simultaneously averting protein misfolding. However the potential activity is often limited to a certain extent depending on the type of interacting protein.6 This ability to alleviate protein misfolding has stimulated researchers to proceed for de novo design of these chemical chaperones. It is hypothesized that chemical chaperones being lower solvents for protein backbone render protein in its native state.7 Industrially these protein stabilizers find use in thwarting aggregation of recombinant protein8 and in preserving enzyme activity both in solution and freeze dried state.9 Protein stabilizers are thought to build up in cytoplasm raising osmotic pressure against environmental water stresses, enhancing protein stability.10 Osmolytes such as trehalose have the property to envelope proteins increasing hydrogen bonding between proteins and water molecules.11 Research in last few decades has witnessed the potential of these chaperones in countering the protein associated pathologies.12 Parkinson's disease is a slow progressive disorder affecting the nervous system afflicting manifestations in the motor function of patients, initiating from the accumulation of alpha-synuclein aggregates in the form of Lewy bodies in the substantia nigra of patients, resulting in degeneration of dopaminergic neurons culminating detrimental effects on motor functions. Selective degeneration of the dopaminergic neurons resulting in impaired levels of dopamine is the characteristic feature of Parkinson's disease. The major constituent of pathological hallmark, Lewy bodies is aggregated alpha-synuclein protein. Parkinson's being a slow progressive disorder augments majority of symptoms when 80% of the dopaminergic neurons are degenerated due to toxic effects of accumulated alpha synuclein. With the current strategies limited to symptomatic relief, quest for newer pharmacological interventions is underway. Protein aggregation is considered one of the hallmarks of this disease resulting in malfunctioning or damage to nerve cells. Molecular chaperones with the ability to decrease the aggregated proteins can be used for the treatment of protein aggregated diseases. The current scientific research is majorly focused on exploring naturally occurring and synthetic protein stabilizers as a complementary or complete therapy to counter the diseases associated with protein misfolding or aggregation, especially when beneficial effects of trehalose have already been reported in context to Huntington's disease13 and Alzheimer's disease.14 Parkinsonism with the known culprit, alpha synuclein holds an intriguing model in investigating osmolytes as potential therapeutic agents. Research on protein stabilizers in context to parkinsonism is speculated to not only present better treatment approaches but as a model for management of other proteopathic neurodegenerative disorders.Investigation into mechanisms underlying chaperone activity of these osmolytes and clearance of aggregation prone biological entities hold significant potential for understanding and amelioration of proteo-pathies. We employed three types of osmolytes i.e. trehalose, sorbitol and xylitol to elucidate their effect on alpha-synuclein aggregation and pathologies associated with parkinsonism using Caenorhabditis elegans as a model.
Materials and methods
C. elegans strains and culture conditions
C. elegans strains used in the study were maintained on a nematode growth medium (NGM) at 22 °C. The food source for these nematodes was Escherichia coli OP50 as described by Brenner 1974 previously.15 The wild type strain used was N2 Bristol and the transgenic strains used in the study were NL5901(Punc-54::alpha-synuclein::YFP + unc-119), TJ356(daf-16p::daf-16a/b::GFP + rol-6), BZ555(Pdat-1::GFP) and DA2123(GFP::LGG-1). These strains were procured from Caenorhabditis Genetics Center, (University of Minnesota, MN, USA).Treatment with compounds and dose optimization
Three protein stabilizers including D-(+)-trehalose (Sigma-Aldrich), D-sorbitol (Sigma-Aldrich) and xylitol (Sigma-Aldrich) were used in the study. These were dissolved in distilled water to obtain two concentrations 5 mM and 10 mM. These molecules were mixed with E. coli OP50 and then seeded onto the NGM plates. With respect to that of control group worms treated with 5 mM dose of all protein stabilizers showed no reduction in alpha-synuclein levels but the effect of 10 mM concentration was significant on the protein levels. So, the further studies to check the multi-factorial aspects of Parkinson's were carried out using 10 mM concentration.Estimation of alpha-synuclein aggregation using fluorescence microscopy
To assess alpha-synuclein aggregation transgenic strain NL5901 was used. This transgenic strain expresses alpha-synuclein protein tagged with yellow fluorescent protein (YFP) in the body wall muscle. The eggs were isolated using hypochlorite treatment and added onto NGM plates seeded with three compounds like trehalose, sorbitol and xylitol. After 48 h of treatment adult worms were washed thrice with M9 buffer to remove adhering bacteria and transferred to microscopy glass slides with agar pads (2% agarose). For immobilization worms were mounted using sodium azide (100 mM) and then covered with a cover slip. The aggregates were monitored using upright fluorescence microscope (Carl-Zeiss). The exposure time was same for all the worms photographed. The mean fluorescence intensity was measured in both control and treated groups by taking six worms from each group using Image-J analysis.Measurement of reactive oxygen species (ROS)
The quantitative measurement of ROS was done to study the extent of oxidative stress in control and treated worms. The method was followed as described previously with slight modifications.16 After 48 h, worms were washed with M9 buffer and then with phosphate buffer saline (PBS). Then, approximately 100 worms from each treatment group were transferred to each well of black plates containing 100 μl PBS. To this, 100 μM stock of 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; Invitrogen) was added to get the final concentration of 50 μM. Fluorescence was taken immediately after adding H2DCFDA and after one hour of addition using fluorimeter. Initial readings were subtracted from the final readings and fluorescence per 100 worms was calculated. The experiment was conducted in triplicate sets.Effect on lipid content
Nile red (Invitrogen) is a lipid specific dye dissolved in acetone (0.5 mg mL−1) to prepare stock solution. The stock is then further diluted (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250) and mixed with E.coli OP50.17 For the treatment with these compounds, Nile red was added to E.coli OP50 mixed with trehalose, sorbitol and xylitol. Embryos were added to these treatment plates and after 48 h adult worms were washed thrice with M9 buffer and transferred to microscopy glass slides with agar pads (2% agarose). For mounting 100 mM sodium azide (Sigma, cat no. 71
250) and mixed with E.coli OP50.17 For the treatment with these compounds, Nile red was added to E.coli OP50 mixed with trehalose, sorbitol and xylitol. Embryos were added to these treatment plates and after 48 h adult worms were washed thrice with M9 buffer and transferred to microscopy glass slides with agar pads (2% agarose). For mounting 100 mM sodium azide (Sigma, cat no. 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 289) for immobilization was used and then overlayed with the cover slip. These immobilized worms were imagined done using Zeiss Axioplan fluorescence microscopy (Rhodamine channel). All worms were photographed at the same exposure time.
289) for immobilization was used and then overlayed with the cover slip. These immobilized worms were imagined done using Zeiss Axioplan fluorescence microscopy (Rhodamine channel). All worms were photographed at the same exposure time.
Motility assay
Motility assay was done to check the thrashing pattern of nematodes as they exhibit a typical thrashing behavior when placed in any liquid. The thrashing pattern depends upon adequate excitatory neurotransmitter content and if any alteration in the excitatory neurotransmitter content, the thrashing behavior of the worm changes.18 Worms from control and treated groups (ten from each group) were transferred to a clean glass slide containing a drop of M9 buffer. A single worm was placed in the drop of buffer and allowed to stabilize followed by the counting of the number of thrashes per 30 seconds using stereo-zoom microscope (Leica, EZ4D). One thrash was defined as complete bending of the body one way to the outermost angle and back to the initial posture. The numbers of thrashes (mean + SE) for each group were evaluated.Estimation of dopamine content using repulsion assay
An established behavioral assay using 1-nonanol is employed to study the dopamine associated effects in nematodes. The assay described earlier was employed with minor modifications to study the response of control and treated groups against 1-nonanol.19 The neurotransmitter dopamine (DA) has an important role in the motor functions of C. elegans in response to food and repellents. Any alteration in the levels of DA leads the nematodes to respond differently to volatile attractants or repellents. In this assay, after 48 h worms were washed thrice with M9 buffer and subjected to 1-nonanol treatment by placing a drop of 1-nonanol near the head of a worm placed in NGM plate. The quick repulsion response is observed in worms with normal dopamine content and in case of altered dopamine, worms show delayed repulsion behavior. We recorded the response time of worms in a batch of ten subjects. The mean response time ± SE for each group was evaluated.Expression of LGG-1 to check autophagy
LGG-1 encodes the ortholog of Saccharomyces cerevisiae Atg8p and mammalian MAP-LC3 in C. elegans. LGG-1 is predicted to be involved in the degradation of cellular components by autophagy.20 The effect of various treatments on LGG-1 expression was checked by using DA2123 strain that expresses GFP::LGG-1. After 48 h of treatment, GFP expression was monitored in early adult worms after washing the worms with M9, immobilizing the worms using 100 mM sodium azide and mounting on agar padded glass slides. GFP-positive punctate regions were visualized in lateral hypodermal seam cells using the fluorescence microscope (Carl Zeiss).Lifespan assay
Embryos isolated with hypochlorite treatment were added to control and treatment plates and 48 hours, the worms were picked and transferred to new treatment plates using the platinum loop. The worms were transferred to fresh plates daily and the numbers of surviving, dead and missing worms were counted until the death of last worm. Worms were considered dead when they did not respond to a mechanical stimulus. Results of survival assays were analyzed using the Kaplan–Meier analysis. The experiment was done in duplicate sets.RNA isolation, cDNA synthesis, and quantitative real-time PCR
For total RNA isolation, after 48 h of treatment adult age-synchronized animals were washed using 0.1% diethylpyrocarbonate (DEPC) water. Total RNA was extracted using Trizol reagent (Invitrogen, Life Technologies) according to the manufacturer's instructions. After washing worms were crushed in Trizol reagent followed by an addition of 200 μl of chloroform per 1 ml of Trizol and centrifuged. 500 μl of chilled isopropanol was used to precipitate RNA, collecting gently the aqueous phase. RNA pellet was washed twice with 75% ethanol (chilled) and finally dissolved in 15 μl of 0.1% DEPC water and RNA was quantified using Nanodrop. For cDNA synthesis, 1 μg of total C. elegans RNA in a 96 well thermal cycler using high capacity cDNA synthesis kit (Applied Biosystems) according to manufacturer's protocol.Quantitative real-time (qRT-PCR) studies were done using Light Cycler 480 machine (Roche Diagnostics, Germany) using equal amount of cDNA template (125 nano molar per reaction). Differential expression was calculated by the 2−ΔΔCT method. Gpd-1 was used as internal control and used to normalize ratios between samples.21 Oligonucleotide sequences used for qRT-PCR orientation (5′ → 3′).
| Gene name | Forward | Reverse |
|---|---|---|
| Bec-1 | AGG AGC TGG AGC AAC AGT TGA AGA | ATA TTG ACG TTC GGC TTC CAG CGA |
| Lgg-1 | AAC AAC TTT GAG AAG CGT CGT GCC | ATC TTC TGG ACG AAG TTG GAT GCG |
| Vps-34 | TGG ATC CCT TTG CAT CAC GAC GTA | CGA AAC AAT CCC AAC ACC ACC GTT |
| Epg-8 | ACG AAA CTC GAA GAG GAA GTC GCT | AGC TCT GAA TCG TTC ACA ACT GCC |
| Hsp-60 | AAG GAT ATG GGA ATT GCG ACG GGA | TGT GCT CGA TTC GCT TCT CGA TCT |
| Hsp-4 | AAC TTC GAT GTC ACC GGA ATC CCA | ATC ATG CGC TCG ATG TCT TCT GGA |
| Gpd-1 | AAA GTC ATT CCG GAG CTG AAC GGA | AGC GGC CTT GAC TAC CTT CTT GAT |
Statistical analysis
Statistical analysis was done using SPSS-15. To calculate significance independent t-test and ANOVA were used, wherever applicable. Kaplan–Meier test was used to calculate mean life span and plot lifespan curves.Results
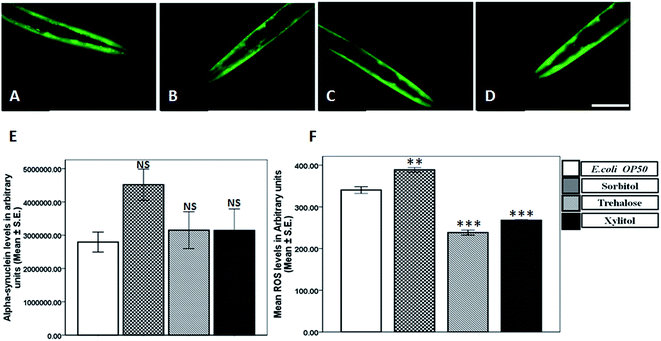
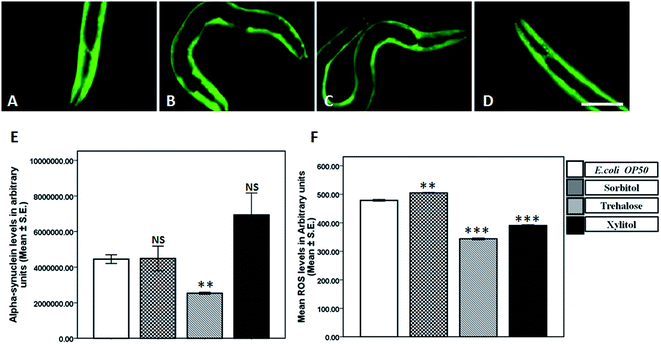
Treatment with 5 mM concentration of sorbitol increased the alpha-synuclein aggregation whereas 10 mM trehalose significantly reduced the alpha-synuclein aggregation in NL5901 worms
Alpha synuclein aggregation is the major manifestation in Parkinson's disease, leading to demise of dopaminergic neurons in substantia nigra of PD patients. In order to elucidate the effect of protein stabilizers on synucleinopathies we subjected the worms expressing alpha-synuclein to 5 mM concentration of sorbitol, trehalose and xylitol. In comparison to that of control (Fig. 1A), no alteration in alpha-synuclein levels was observed upon treatment with trehalose (Fig. 1C) and xylitol (Fig. 1D), where as a significant increase in alpha-synuclein levels was observed upon treatment with sorbitol (Fig. 1B). The results so obtained from fluorescence microscopy were quantified using Image J (Fig. 1E) and in comparison to the fluorescence observed in control, OP50 (2.79 ± 0.30 × 106) there was increase in alpha-synuclein levels upon treatment with 5 mM sorbitol (4.51 ± 0.47 × 106), whereas no alteration was observed upon treatment with 5 mM trehalose (3.15 ± 0.55 × 106) and 5 mM xylitol (3.14 ± 0.64 × 106).After assessing effect of 5 mM concentration of protein stabilizers on alpha-synuclein levels, we determined the effect of 10 mM concentration of protein stabilizers on alpha-synuclein levels. In comparison to control worms treated with E. coli OP50 (Fig. 2A), we observed a significant decrease upon treatment with 10 mM trehalose (Fig. 2C), whereas no alteration in alpha-synuclein levels was observed upon treatment with 10 mM sorbitol (Fig. 2B) and 10 mM xylitol (Fig. 2D). The fluorescent images were further assessed semi-quantitatively using Image J. In comparison to the levels of alpha-synuclein levels in OP50 (4.53 ± 0.24 × 106), we observed a significant decrease upon treatment with 10 mM trehalose (2.53 ± 0.06 × 106), whereas a significant increase in alpha-synuclein levels was observed upon treatment with 10 mM xylitol (6.93 ± 0.12 × 106). However no alteration in alpha-synuclein levels was observed upon treatment with 10 mM sorbitol (4.48 ± 0.69 × 106) (Fig. 2E).
Treatment with 5 mM and 10 mM trehalose and xylitol decreased the ROS levels in wild-type worms
Toxic alpha-synuclein aggregates tend to cause neuronal damage in substantia-nigra of PD patients. Increased ROS has been considered as a reason and consequence of neuronal damage in parkinsonism. In order to investigate the effect on ROS levels we employed H2DCFDA, a cell permeable dye which reacts with reactive oxygen species to generate fluorescence. In comparison to the ROS levels observed in E. coli OP50 (340.35 ± 8.15), we observed a significant increase in ROS levels upon treatment with 5 mM sorbitol (388.88 ± 5.34), where as a significant decrease was observed upon treatment with 5 mM trehalose (238.34 ± 5.98) and 5 mM xylitol (267.77 ± 1.46), implicating beneficial effects of trehalose and xylitol on ROS levels (Fig. 1F).In line with the effects observed in 5 mM treatment groups we also determined the effect of higher concentration (10 mM) of protein stabilizers on ROS levels in wild type worms. In comparison to the ROS levels in OP50 (478.66 ± 3.76), we observed a significant decrease in ROS levels in worms treated with 10 mM trehalose (343.93 ± 3.49) and 10 mM xylitol (390.53 ± 2.74), whereas a significant increase in ROS levels was exhibited by the worms treated with 10 mM sorbitol (504.76 ± 1.81) (Fig. 2F).
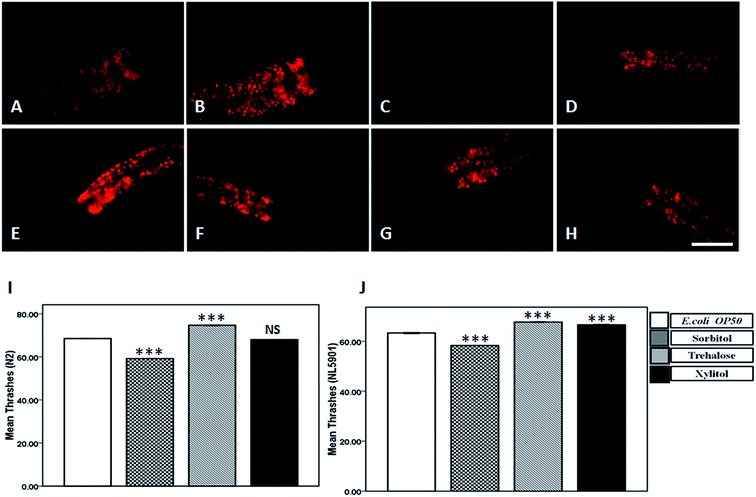
Treatment with 10 mM trehalose reduced lipid-levels in wild type strain N2 whereas there was no change in lipid-content in transgenic NL5901 worms upon treatment with trehalose, sorbitol and xylitol
We studied the effect of 10 mM concentration of protein stabilizers on lipid content using Nile red staining in both N2 and NL5901 worms. In comparison to the optimum lipid-levels in E. coli OP50 (Fig. 3A) in wild type worms, we observed a significant decrease in lipid-content in worms treated with trehalose (Fig. 3C), whereas sorbitol (Fig. 3B) and xylitol (Fig. 3D) did not alter lipid-levels. The worms subjected to 10 mM xylitol were relatively thin in comparison to control.In comparison to the lipid-content in N2 worms we observed a significant increase in lipid-content in NL5901 worms expressing alpha-synuclein. However, in comparison to the levels of lipid-content in control (Fig. 3E) in NL5901 worms we did not observe any alteration in lipid content upon treatment with 10 mM concentration of trehalose (Fig. 3F), sorbitol (Fig. 3G) and xylitol (Fig. 3H).
NL5901 worms showed improved motility upon treatment with trehalose and xylitol, which was limited to trehalose only in case of wild type worms
Parkinsonism is associated with impaired motor function. In order to study the effect of protein stabilizers on motility we performed thrashing assay for worms treated with 10 mM concentration of protein stabilizers against E. coli OP50 treated worms (68.50 ± 0.05). We observed a significant increase in number of thrashes in worms subjected to 10 mM trehalose (74.66 ± 0.23) whereas worms treated with 10 mM sorbitol exhibited decrease in number of thrashes (59.16 ± 0.08). The alteration in number of thrashes shown by 10 mM xylitol (68.00 ± 0.05) however was non-significant with respect to control, E. coli OP50 (Fig. 3I). In NL5901, as compared to the number of thrashes in OP50 (63.33 ± 0.29), we observed a significant increase in number of thrashes in worms treated with 10 mM trehalose (67.70 ± 0.15) and 10 mM xylitol (66.60 ± 0.17). Treatment with 10 mM sorbitol (59.16 ± 0.08) exhibited decrease in number of thrashes as was the case in wild-type worms (Fig. 3J).Trehalose treatment increased the dopamine levels as indicated by repulsion assay in wild-type and NL5901 worms
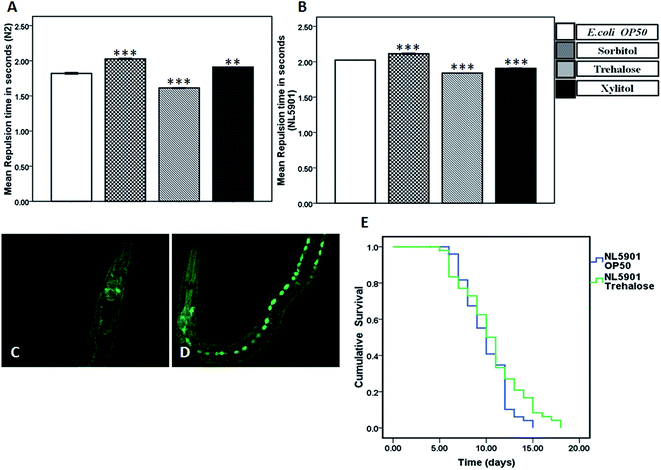
We studied the effect of protein stabilizers on dopamine levels, a major contributor to the neuro-motor function of body in both wild-type and transgenic NL5901worms by employing an indirect assay called repulsion assay. The nonanol assay is a chemo-taxis based evaluation; relying on the fact that 1-nonanol is a chemo-repellant which causes worms to exhibit a strong repulsive behavior upon olfaction of nonanol. As a rule, greater repulsion time indicates lower dopamine levels and vice versa. We observed a significant decrease in repulsion time in worms treated with 10 mM trehalose (1.61 ± 0.00) as compared to that of control, E. coli OP50 (1.82 ± 0.01) implicating an increase in dopamine levels upon treatment with 10 mM trehalose. Conversely, a significant increase in repulsion time indicative of reduced dopamine levels was observed in wild type worms subjected to 10 mM sorbitol (2.02 ± 0.00) and 10 mM xylitol (1.91 ± 0.00) (Fig. 4A).In NL5901 worms, compared to the repulsion time in control (E. coli OP50) (2.02 ± 0.00), we observed a significant decrease in repulsion time in worms treated with 10 mM trehalose (1.84 ± 0.00), implicating an increase in dopamine levels. The treatment groups 10 mM sorbitol (2.11 ± 0.01) and 10 mM xylitol (1.90 ± 0.00) however exhibited opposite trend indicating reduced dopamine levels (Fig. 4B).
Trehalose treatment induced autophagy via increased expression of LGG-1
LGG-1 is an autophagy marker in C. elegans that shows diffused or punctate expression in cells. A large number of punctate structures are representative of increased autophagy. We observed a significant increase in punctate LGG-1::GFP structures, especially in seam cells upon treatment with trehalose (Fig. 4D) as compared to control (Fig. 4C), implicating an increase in autophagy.Trehalose treatment increased the lifespan of the transgenic NL5901 worms
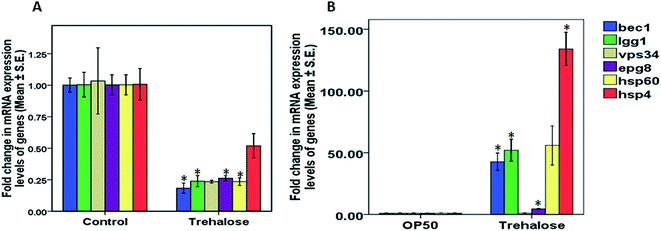
After ascertaining the effect of 10 mM trehalose on life span in wild-type worms we checked the effect of trehalose treatment on the modulation of lifespan in NL5901 worms. Contrary to the results observed in wild-type worms, we observed an increase in life span in worms treated with trehalose (10.60 ± 0.49) as compared to that of control, OP50 (9.96 ± 0.33) in NL5901 strain (Fig. 4E). These results implicated that higher concentration of trehalose was beneficial for longevity in disease condition.Treatment with trehalose up-regulated the mRNA expression of genes associated with autophagy and heat-shock in transgenic NL5901 worms but not in wild type N2 strain
Protein stabilizers are said to possess chaperonic activity and are hence considered as potential agents with property to alleviate mis-folded proteins. In order to detect any possible role of the inherent autophagic and chaperonic system by trehalose we performed real time PCR to check the levels of mRNA expression in both wild-type worms and NL5901 worms treated with 10 mM trehalose.Discussion
Protein misfolding and aggregation are a cause of majority of neurodegenerative disorders like Alzheimer's and Parkinson's. Protein stabilizers possess the potential to inhibit protein aggregation or orchestrate the correct folding of proteins. Trehalose is a simple naturally occurring disaccharide of glucose found in diverse organisms including plants, fungi, bacteria and yeasts and is known to avert denaturation, misfolding and aggregation of proteins. Trehalose inhibits aggregation of proteins involved in polyglutamine and polyalanine disorders.22 Sorbitol has been reported to stabilize protein aggregation through direct interaction. Xylitol is reported to protect proteins from heat-induced denaturation in aqueous solutions.23 Similar to trehalose, xylitol protects various micro-organisms and biological macromolecules including proteins from inactivation or viability loss during freeze-drying and freeze-thawing24 dependent on various physical properties like molecular mobility and crystallinity. In this study, we have tried to unleash the potential of rationally selected osmolytes like trehalose, sorbitol and xylitol in amelioration of synucleinopathies in C. elegans model of Parkinson's disease. The exact mechanism of these osmolytes and whether they have any effect on the inherent chaperonic machinery, made this study quite intriguing. We carried out our initial studies using 5 mM concentration of selected osmolytes i.e. trehalose, sorbitol and xylitol. We observed marginal increase in alpha-synuclein aggregation upon treatment with sorbitol with some reduction in ROS levels upon treatment with trehalose and xylitol in wild-type worms. Since the results observed upon treatment with 5 mM osmolytes were not significantly compelling to proceed for further studies, we studied the effect of higher concentration of osmolytes i.e. 10 mM, where we observed significant reduction in alpha-synuclein levels upon treatment with 10 mM trehalose. Paradoxically a significant increase in protein aggregation was observed upon treatment with 10 mM xylitol. The results implicated effect of trehalose in countering the toxic protein load specific to Parkinson's disease. Altered lipid levels are considered as one of the characteristics in PD patients, our next aim was to elucidate the effect of osmolytes on lipid-content. Significant reduction in lipid-levels upon treatment with 10 mM trehalose specific to wild-type worms only. These results substantiated the reports about altered lipid-levels in PD patients along with lipid lowering properties of osmolytes. Dopaminergic neuronal damage being caused due to increased ROS has been a major manifestation in parkinsonism.25 In order to ascertain the effect of these osmolytes on ROS levels we elucidated the ROS content and observed a significant reduction in ROS levels upon treatment with 10 mM trehalose, whereas an increase in ROS levels was observed in worms treated with 10 mM sorbitol. These results not only indicated decreased ROS as a possible protective mechanism in case of trehalose but also posed a probable clue for deleterious effects of higher concentration of sorbitol, which was very much in concurrence with the results observed in the previous studies employing higher concentration of sorbitol. Damage to motor neurons has been linked to impaired motor function in Parkinsonism.26 We tried to assess the effect of 10 mM concentration on motility in wild-type and NL5901 worms. We observed a significant increase in number of thrashes upon treatment with 10 mM trehalose in both wild type and NL5901 worms, whereas treatment with 10 mM sorbitol exhibited reduction in motility in both NL5901 and N2 worms. The beneficial effects of 10 mM xylitol on motility were however limited to NL5901 worms only. In continuation to the effect on motor function we also studied the effect of osmolytes on dopamine levels employing an indirect repulsion assay. In this assay, repulsion time in response to 1-nonanol is inversely proportional to the dopamine levels. Higher repulsion time is representative of lower dopamine levels and vice-versa. We observed a significant increase in dopamine levels as indicated by lower repulsion time in case of wild type and NL5901 worms treated with 10 mM trehalose, whereas a significant decrease in dopamine levels was observed in case of N2 and NL5901 worms subjected to 10 mM sorbitol and xylitol. After assessing the effects of osmolytes on the above parameters related to parkinsonism we observed that trehalose at higher concentrations was most potent of all osmolytes in countering PD related manifestations represented in C. elegans. We selected trehalose for its effect on life span, where we observed that 10 mM trehalose led to a significant increase in lifespan in NL5901 worms expressing alpha-synuclein. As there are studies which suggest that trehalose induces autophagy so we also checked the effect of trehalose on autophagy and observed relatively increased punctate LGG-1::GFP structures indicating amassed autophagy. We next aimed to determine the protective effect of trehalose on mRNA expression of genes related to autophagy and heat-shock system in both wild-type and NL5901 worms. We observed a significant down-regulation of genes, bec-1, lgg-1, vps-34, hsp-60 and hsp-4 in wild type worms, which might be attributed to the autophagic or protein stabilizing potential of trehalose. Since trehalose treatment is expected to reduce the proteotoxic load within the biochemical milieu causing down-regulation of autophagic and chaperonic machinery in a kind of feedback inhibitory manner. As speculated in the NL5901 worms, with significant amount of proteotoxic load a significant up-regulation in expression of bec-1, lgg-1, epg-8, hsp-60 and hsp-4 was observed implicating that trehalose, besides possessing inherent protein stabilizing potential works in collaboration with the cellular machinery, resulting in up-regulation of autophagy and chaperonic genes to counter the toxic build up of misfolded and aggregated proteins. The results from this study implicate the protective role of trehalose treatment in reducing mutant protein via inherent and cellular mechanisms along with exerting beneficial effects towards other manifestations such as dopaminergic and motility functions.Our findings are in line with the reported beneficial effects of trehalose in various protein aggregation related neurological disorders, with existence of sufficient research literature corroborating our results. Ameliorative potential of trehalose on MPTP model of parkinsonism in mice has recently been reported, where trehalose treatment was found to mitigate microglial activation and astrocytic hypertrophy, besides sufficing with protection to micro vessels and endothelial cells against the toxic effects of MPTP.27 Trehalose has also been known for its inhibitory effects on alpha synuclein fibril formation.28 Besides possessing inherent protein stabilization properties trehalose has also been reported to enhance autophagy, mitigating aggregation of mutant huntingtin and alpha synuclein protein in an mTOR independent manner.29
Conclusion
Our findings present evidence regarding the potential role of trehalose in ameliorating synucleinopathies and associated manifestations in parkinsonism, which are in concurrence with the reported chaperonic activity of protein stabilizers. We conclude that further research towards comprehending the underlying mechanisms arising from direct or indirect protein stabilizer–protein association and synthesis of novel protein stabilizers will not only buildup understanding about the mode of action of osmolytes but will also open up avenues for therapeutic attenuation of proteopathic disorders through molecular chaperones. Research on the subject will be required towards establishing their mechanism of action and suitability of such intervention, possibly along with existing therapies that address other important factors of the disease.Competing interests
The authors have declared that no competing interests exist.Author contributions
Conceived and designed the experiments: AN and SK, performed the experiments: SK analyzed the data: SK; wrote and edited the paper: SK, AN.Acknowledgements
Nematode strains used in this work were provided by the C. elegans Genetics Center (CGC) University of Minnesota, MN, USA, which is funded by the NIH National Center for Research Resources (NCRR). SK was supported by fellowship from Council of Scientific and Industrial Research (CSIR) (ref. 113177/2K12/1). AN gratefully acknowledges funding from Department of Science and Technology, Govt. of India, under Cognitive Science Research Initiative (SR/CSI/17/2012(G)). CDRI Communication number is 9076.References
- B. Elliott, R. S. Haltiwanger and B. Futcher, Synergy between trehalose and Hsp104 for thermotolerance in Saccharomyces cerevisiae, Genetics, 1996, 144, 923–933 CAS.
- M. A. Singer and S. Lindquist, Multiple effects of trehalose on protein folding in vitro and in vivo, Mol. Cell, 1998, 1, 639–648 CrossRef CAS PubMed.
- R. I. Viner and J. S. Clegg, Influence of trehalose on the molecular chaperone activity of p26, a small heat shock/alpha-crystallin protein, Cell Stress Chaperones, 2001, 6, 126–135 CrossRef CAS PubMed.
- A. L. Bulman and H. C. M. Nelson, Role of trehalose and heat in the structure of the C-terminal activation domain of the heat shock transcription factor, Proteins: Struct., Funct., Genet., 2005, 58, 826–835 CrossRef CAS PubMed.
- L. K. Conlin and H. C. M. Nelson, The natural osmolyte trehalose is a positive regulator of the heat-induced activity of yeast heat shock transcription factor, Mol. Cell. Biol., 2007, 27, 1505–1515 CrossRef CAS PubMed.
- A. De Marco, Molecular and chemical chaperones for improving the yields of soluble recombinant proteins, Methods Mol. Biol., 2011, 705, 31–51 CrossRef CAS PubMed.
- R. Dandage, A. Bandyopadhyay, K. Saxena, V. Dalal and A. Das, Classification of chemical chaperones based on their effect on protein folding landscapes, ACS Chem. Biol., 2015, 10, 813–820 CrossRef CAS PubMed.
- L. Kai, E. Orbán, E. Henrich, D. Proverbio, V. Dötsch and F. Bernhard, Co-translational stabilization of insoluble proteins in cell-free expression systems, Methods Mol. Biol., 2015, 1258, 125–143 CrossRef CAS PubMed.
- J. K. Kaushik and R. Bhat, Why is trehalose an exceptional protein stabilizer? An analysis of the thermal stability of proteins in the presence of the compatible osmolyte trehalose, J. Biol. Chem., 2003, 278, 26458–26465 CrossRef CAS PubMed.
- R. S. Rajan, K. Tsumoto, M. Tokunaga, H. Tokunaga, Y. Kita and T. Arakawa, Chemical and pharmacological chaperones: application for recombinant protein production and protein folding diseases, Curr. Med. Chem., 2011, 18, 1–15 CrossRef CAS PubMed.
- S. E. Stidham, S. L. Chin, E. L. Dane and M. W. Grinstaff, Carboxylated glucuronic poly-amido-saccharides as protein stabilizing agents, J. Am. Chem. Soc., 2014, 136, 9544–9547 CrossRef CAS PubMed.
- L. Cortez and V. Sim, The therapeutic potential of chemical chaperones in protein folding diseases, Prion, 2014, 8, 197–202 CrossRef CAS.
- M. Tanaka, Y. Machida, S. Niu, T. Ikeda, N. R. Jana and H. Doi, et al., Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease, Nat. Med., 2004, 10, 148–154 CrossRef CAS PubMed.
- R. Liu, H. Barkhordarian, S. Emadi, B. P. Chan and M. R. Sierks, Trehalose differentially inhibits aggregation and neurotoxicity of beta-amyloid 40 and 42, Neurobiol. Dis., 2005, 20, 74–81 CrossRef CAS PubMed.
- S. Brenner, The genetics of Caenorhabditis elegans, Genetics, 1974, 77, 71–94 CAS.
- M. Artal-Sanz and N. Tavernarakis, Prohibitin couples diapause signalling to mitochondrial metabolism during ageing in C. elegans, Nature, 2009, 461, 793–797 CrossRef CAS PubMed.
- K. Ashrafi, F. Y. Chang, J. L. Watts, A. G. Fraser, R. S. Kamath and J. Ahringer, et al., Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes, Nature, 2003, 421, 268–272 CrossRef CAS PubMed.
- S. D. Buckingham and D. B. Sattelle, Fast, automated measurement of nematode swimming (thrashing) without morphometry, BMC Neurosci., 2009, 10, 84 CrossRef PubMed.
- C. I. Bargmann, E. Hartwieg and H. R. Horvitz, Odorant-selective genes and neurons mediate olfaction in C. elegans, Cell, 1993, 74, 515–527 CrossRef CAS PubMed.
- K. Asanuma, I. Tanida, I. Shirato, T. Ueno, H. Takahara and T. Nishitani, et al., MAP-LC3, a promising autophagosomal marker, is processed during the differentiation and recovery of podocytes from PAN nephrosis, FASEB J., 2003, 17, 1165–1167 CAS.
- K. J. Livak and T. D. Schmittgen, Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method, Methods, 2001, 25, 402–408 CrossRef CAS PubMed.
- J. E. Davies, S. Sarkar and D. C. Rubinsztein, Trehalose reduces aggregate formation and delays pathology in a transgenic mouse model of oculopharyngeal muscular dystrophy, Hum. Mol. Genet., 2006, 15, 23–31 CrossRef CAS PubMed.
- S. N. Timasheff, The control of protein stability and association by weak interactions with water: how do solvents affect these processes?, Annu. Rev. Biophys. Biomol. Struct., 1993, 22, 67–97 CrossRef CAS PubMed.
- S. N. Timasheff, Control of protein stability and reactions by weakly interacting cosolvents: the simplicity of the complicated, Adv. Protein Chem., 1998, 51, 355–432 CrossRef CAS PubMed.
- V. Dias, E. Junn and M. M. Mouradian, The role of oxidative stress in parkinson's disease, J. Parkinson's Dis., 2013, 3, 461–491 CAS.
- W. Dauer and S. Przedborski, Parkinson's disease: Mechanisms and models, Neuron, 2003, 39, 889–909 CrossRef CAS PubMed.
- S. Sarkar, S. Chigurupati, J. Raymick, D. Mann, J. F. Bowyer and T. Schmitt, et al., Neuroprotective effect of the chemical chaperone, trehalose in a chronic MPTP-induced Parkinson's disease mouse model, NeuroToxicology, 2014, 44, 250–262 CrossRef CAS PubMed.
- W. B. Yu, T. Jiang, D. M. Lan, J. H. Lu, Z. Y. Yue and J. Wang, et al., Trehalose inhibits fibrillation of A53T mutant alpha-synuclein and disaggregates existing fibrils, Arch. Biochem. Biophys., 2012, 523, 144–150 CrossRef CAS PubMed.
- S. Sarkar, J. E. Davies, Z. Huang, A. Tunnacliffe and D. C. Rubinsztein, Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein, J. Biol. Chem., 2007, 282, 5641–5652 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |