Cosolvent-free nanocasting synthesis of ordered mesoporous g-C3N4 and its remarkable photocatalytic activity for methyl orange degradation†
Xiaochun Gaoa,
Xuejiao Jiaoc,
Lanchun Zhanga,
Wencai Zhua,
Xiaohong Xua,
Houyi Ma*a and
Ting Chen*b
aKey Laboratory of Colloid and Interface Chemistry of State Education Ministry, School of Chemistry and Chemical Engineering, Shandong University, Jinan 250100, P. R. China. E-mail: hyma@sdu.edu.cn; Fax: +86-531-88564464; Tel: +86-531-88364959
bSchool of Science, Shandong Jianzhu University, Jinan 250101, P. R. China. E-mail: chenting@sdjzu.edu.cn; Tel: +86-531-86361596
cState Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130023, P. R. China
First published on 3rd September 2015
Abstract
Ordered mesoporous g-C3N4 (ompg-C3N4) was synthesized in an aqueous solution via a green cosolvent-free nanocasting route by replacing the extremely virulent cyanamide with the environment-friendly starting material dicyandiamide (DCDA) for the first time. The well-defined cashew-shaped ompg-C3N4 was obtained when the weight ratio of DCDA to mesoporous silica SBA-15 was controlled at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the nanocasting process. The excellent photocatalytic performance towards the degradation of methyl orange (MO), a hazardous dye, was evaluated under visible light irradiation and the degradation rate constant of ompg-C3N4 was determined to be 30 times as high as that of bulk g-C3N4. The significantly enhanced photodegradation activity of ompg-C3N4 is mainly attributed to the ordered mesopores inside individual particles, which accelerate the adsorption of the dye onto the catalyst surface and shorten the diffusion path of free carriers from the bulk phase to the surface, thereby enhancing the separation efficiency of photogenerated electrons and holes. In addition, a possible MO photodegradation mechanism was proposed based on the results of the control experiments in the presence and absence of scavengers.
1 in the nanocasting process. The excellent photocatalytic performance towards the degradation of methyl orange (MO), a hazardous dye, was evaluated under visible light irradiation and the degradation rate constant of ompg-C3N4 was determined to be 30 times as high as that of bulk g-C3N4. The significantly enhanced photodegradation activity of ompg-C3N4 is mainly attributed to the ordered mesopores inside individual particles, which accelerate the adsorption of the dye onto the catalyst surface and shorten the diffusion path of free carriers from the bulk phase to the surface, thereby enhancing the separation efficiency of photogenerated electrons and holes. In addition, a possible MO photodegradation mechanism was proposed based on the results of the control experiments in the presence and absence of scavengers.
Introduction
The widespread disposal of industrial wastewater containing organic dyes onto land and water bodies has led to severe environmental pollution on a global scale.1–4 Dyes have adverse effects on animal and human health, including (partial) toxicity, mutagenicity and carcinogenicity.5 Semiconductor-based photocatalytic degradation of organic pollutants is one of the most promising, eco-friendly environmental control techniques and thus has become the focus of intensive fundamental and applied research in recent years.6–11 Among the existing photocatalysts, nano-TiO2 has received the most widespread attention because of its chemical inertness, resistance to photocorrosion, low cost, and non-toxicity.12–14 However, the key problem that hinders its extensive application is its wide band gap of 3.2 eV, indicating that TiO2 can only absorb about 3–5% of UV-light in the ultraviolet region.15 In this regard, creating efficient, sustainable photocatalysts with excellent visible-light responses has been identified as the major challenge in this research field.Graphitic carbon nitride (g-C3N4) has attracted a great deal of attention as a promising metal-free photocatalyst for the degradation of organic pollutants since its superior visible-light photocatalytic activity in hydrogen production was first discovered by Wang's group.16 The appropriate positions of its conduction band (CB) and valence band (VB),17 which make it fairly suitable for sustainable utilization of solar energy, such as water splitting,18 organic photosynthesis,19 and environmental remediation.20 Unlike the multicomponent oxide, metal oxide, and sulfide photocatalysts, such as BiVO4, TiO2, and CdS, g-C3N4 possesses high stability with respect to chemical (e.g., acid and base), thermal (<873 K), and photochemical attack owing to its tri-s-triazine ring structure and high degree of condensation.21,22 Although g-C3N4 holds great potential in solar energy conversion, the high recombination rate of its photogenerated electron–hole pairs and low specific surface area (<10 m2 g−1) restrict the improvement of photocatalytic efficiency, which consequently leads to inferior photocatalytic performance.
Fabricating advanced nanostructures is demonstrated to be one of the most effective ways to enhance the photocatalytic activity of g-C3N4. Porous structured g-C3N4 materials are especially attractive (photo)catalysts, which have been proven to be chemically productive by enhancing mass transfer, offering more surface sites, and improving light harvesting. Moreover, the recombination of photogenerated electron–hole pairs inside the high-surface area material can be greatly suppressed because of the short diffusion path of free carriers from the bulk to the surface.23 This finding has motivated significant research into the synthesis of mesoporous g-C3N4 (mpg-C3N4) materials.24–26 Typically, mpg-C3N4 materials are synthesized by hard templating, using nanostructured silica27–30 or commercially available aluminum oxide as template31 and liquid monomer cyanamide as precursor. Wang and coworkers32,33 reported the synthesis of ordered mesoporous g-C3N4 through the nanocasting pathway, and found that hydrogen production increased by 5 to 8 times, compared to the bulk material. Antonietti et al.34 re-prepared a series of 12 nm-sized mpg-C3N4 materials with high N content (C/N = 0.71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, close to the theoretical molar ratio of g-C3N4). The resulting material possessed high surface area and high catalytic activity in Friedel–Crafts reaction. Disappointingly, the most widely used precursor, cyanamide, is virulent, explosive, and expensive, which limits the production of ompg-C3N4. Furthermore, utilization of other precursors, such as guanidinium chloride,35 urea,36 and hexamethylenetetramine37 also afforded low productivities. Thus it is definite necessary to find toxic-free and low-cost precursors, as well as high-yield carbon nitride products.
1, close to the theoretical molar ratio of g-C3N4). The resulting material possessed high surface area and high catalytic activity in Friedel–Crafts reaction. Disappointingly, the most widely used precursor, cyanamide, is virulent, explosive, and expensive, which limits the production of ompg-C3N4. Furthermore, utilization of other precursors, such as guanidinium chloride,35 urea,36 and hexamethylenetetramine37 also afforded low productivities. Thus it is definite necessary to find toxic-free and low-cost precursors, as well as high-yield carbon nitride products.
In this study, we report the synthesis of cashew-like ompg-C3N4 via nanocasting employing SBA-15 as siliceous source and the widely available, low cost, relatively non-toxic dicyandiamide (DCDA) as precursor, without the use of any hazardous organic cosolvent. It is particularly worth mentioning here that we got ompg-C3N4 when the weight ratio of DCDA/SAB-15 was optimized to 2 and the yield of ompg-C3N4 was up to 50% which was much higher than that obtained using cyanamide as precursor.38 Favorable photodegradation of the material for methyl orange (MO) was observed under visible light (λ ≥ 420 nm) irradiation, and the significant improved degradation rate was much higher than that of g-C3N4 based heterojunction material. To the best of our knowledge, the study is the first to use ompg-C3N4 synthesized using DCDA as precursor on the photodegradation of dyes. Excellent photocatalytic performance was demonstrated and the possible mechanism was interpreted based on the experimental results.
Experimental
Preparation of ompg-C3N4 and bulk g-C3N4
The ompg-C3N4 was obtained in a typical synthesis, 1.0 g of SBA-15 (ref. 39) was impregnated in 70 mL of aqueous solution containing 2.0 g of DCDA and sonicated for 2 h. The resulting mixture was then heated to 80 °C for 24 h to remove the water, and finally calcined under nitrogen atmospheres at 550 °C for 4 h at a heating rate of 2.3 °C min−1. To remove the silica template, the resulting silica-g-C3N4 powders were treated with 2 M NaOH for 24 h, followed by filtration, washing with water and ethanol for several times, and drying at 50 °C. Finally, the desired light-yellow powder, denoted as CN2, was obtained (Fig. 1). A series of optimizing experiments were also carried out with different weight ratios of SBA-15/DCDA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4); and the synthetic products at above-mentioned ratios were denoted as CN1, CN3, CN4, respectively.
4); and the synthetic products at above-mentioned ratios were denoted as CN1, CN3, CN4, respectively.
 | ||
| Fig. 1 A schematic diagram of the synthesis of ompg-C3N4 templated from SBA-15 using DCDA monomer, and its schematic molecular structure. | ||
Bulk g-C3N4 was synthesized by directly heating dicyandiamide according to the reported procedure.40 Typically, 2 g of dicyandiamide powder was put into an alumina crucible with a cover, then heated at a rate of 2.3 °C min−1 to reach a temperature of 550 °C and finally tempered at this temperature for another 4 h.
Characterization of photocatalysts
The crystalline phases of the materials were evaluated by X-ray diffraction using a Bruker D8 diffractometer with Cu Kα radiation (λ = 1.5418 Å) in the range of 2θ = 10–70°. Nitrogen-sorption isotherms were collected using an ASAP 2020 adsorption analyzer (Micromeritics) at liquid nitrogen temperature. X-ray small-angle scattering (SAXS) measurements were carried out on a SAXSess mc2 instrument (Anton Paar), equipped with a high performance CCD detector from Roper Scientific (pixel size 24 μm, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 pattern). The morphology of the samples was observed using a transmission electron microscope (TEM; JEOL JEM 2100). The Fourier transform infrared (FTIR) spectra were recorded in transmission mode from 4000 to 400 cm−1 on a NicoletiS10 FTIR spectrophotometer. X-ray photoelectron spectroscopic (XPS) measurements were performed on an ESCALAB 250 spectrometer equipped with an Al Kα source. Photoluminescence spectra (PL) were obtained using a Hitachi F7000 fluorescence spectrometer with an excitation wavelength of 325 nm. UV-vis diffuse absorption spectra were investigated with a UV-visible spectrometer (UV-3100, Hitachi).
1 pattern). The morphology of the samples was observed using a transmission electron microscope (TEM; JEOL JEM 2100). The Fourier transform infrared (FTIR) spectra were recorded in transmission mode from 4000 to 400 cm−1 on a NicoletiS10 FTIR spectrophotometer. X-ray photoelectron spectroscopic (XPS) measurements were performed on an ESCALAB 250 spectrometer equipped with an Al Kα source. Photoluminescence spectra (PL) were obtained using a Hitachi F7000 fluorescence spectrometer with an excitation wavelength of 325 nm. UV-vis diffuse absorption spectra were investigated with a UV-visible spectrometer (UV-3100, Hitachi).
Photocatalytic activity
The photocatalytic activity of the photocatalysts was evaluated by photocatalytic oxidation of MO dye under visible light irradiation. A 500 W Xe lamp (Lansheng Electronics Ltd, Shanghai) with a 420 nm cutoff filter was used as light source to provide visible light irradiation. The illumination intensity was 20 mW cm−2. Approximately 100 mg of photocatalysts were dispersed into 100 mL of 20 mg L−1 MO solution for photocatalytic examination under magnetic stirring. Prior to light irradiation, the mixture was kept in the dark for 30 min under magnetic stirring to reach adsorption–desorption equilibrium. Solutions were collected every 6 min, centrifuged to remove the catalyst, and then analyzed with a UV-visible spectrometer (UV-3100, Hitachi). Degradation efficiency was calculated using C/C0, where C is the concentration of the remaining dye solution at each irradiated interval, and C0 is the initial concentration.Photoelectrochemical measurements
The photocurrents were measured on a CHI-650D electrochemical workstation (Chen Hua Instrument, Shanghai, China) in a standard three electrode system, in which the C3N4-based electrode was used as working electrode, a large Pt plate was used as counter one, and a saturated calomel electrode (SCE) as reference one. A 500 W Xe arc lamp with a 420 nm cutoff filter was employed as light source. The electrolyte was a 0.5 M Na2SO4 aqueous solution. The working electrodes were fabricated using the following procedure: (i) 20 mg of as-prepared g-C3N4 powders were fully mixed with a certain amount of ethanol solution; (ii) the resulting catalyst slurry was spread on a 15 mm × 20 mm tin-doped indium oxide (ITO) conducting glass; (iii) each electrode was dried at 30 °C under vacuum and calcined at 200 °C for 4 h in sequence.Results and discussion
Structure and morphology characterizations
The crystalline phases of five g-C3N4 samples prepared under different synthetic conditions were identified by XRD measurements. As shown in Fig. 2A, for the silica-free samples, the well-resolved peak at 2θ = 27.6° was a characteristic interplanar stacking peak of conjugated aromatic systems, indexed for graphitic materials as the (002) peak. However, the (002) peaks of CNX (X = 1, 2, 3, 4) samples looked slightly wider than that of bulk g-C3N4, which was attributed to the geometric confinement in the nanosized pore walls after the nanocasting process.30 The interplanar distance was calculated as d = 0.323 nm, which was significantly smaller than that of the crystalline g-C3N4 (d = 0.34 nm).41 The dense structure can be attributed to the localization of the electrons and stronger binding (hydrogen bonding, and van der Waals force) between layers. The other peak at 13.1°, corresponding to the in-plane structural packing motif of tri-s-triazine units, was indexed as the (100) peak.42 Additionally, taking CN2 + SBA-15 as an example among the CNX + SBA-15 samples (Fig. S1, ESI†), the diffraction intensity was much weaker as compared with silica-free CN2 in Fig. 2B. Except or the characteristic peaks at 27.6° and 13.1°, the broad peak at 22° caused by SBA-15 were not observed from XRD patterns of CNX, demonstrating that SBA-15 were removed completely and the lattice structure of g-C3N4 was retained in all CNX materials after the nanocasting modification.To further evaluate the successful replication, the structures and morphologies of SBA-15 and CNX replicas were characterized and compared by performing SAXS, N2 adsorption–desorption and TEM measurements. As shown in Fig. 3, the SAXS pattern of SBA-15 exhibits three well-resolved scattering peaks, indexed as (100), (110), and (200) reflections, revealing a 2D hexagonal pore structure with p6mm symmetry. The most obvious (100) and (110) reflection peaks were only observed in the SAXS pattern of CN2, indicating that the 2D hexagonal mesostructure of SBA-15 was finely preserved in CN2. By contrast, the (100) and (110) peaks corresponding to CN1, CN3, and CN4 were not as sharp as those of CN2, which implied poorer replication. Additionally, a significant shift of d100 (Table 1) to smaller values indicates that a serious shrinkage in structure occurred in CN1, CN3, and CN4 after the template was removed. Based on the shape and position of the scattering peaks, we inferred that well-defined ompg-C3N4 material was obtained only when the weight ratio of DCDA/SBA-15 was optimized to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Its ordered mesostructure greatly favored mass transfer and was responsible for the enhanced photocatalytic activity.
1. Its ordered mesostructure greatly favored mass transfer and was responsible for the enhanced photocatalytic activity.
| Sample | SBET [m2 g−1] | Vporea [cm3 g−1] | d100b [nm] |
|---|---|---|---|
| a Determined at p/p0 = 0.99, where p is the equilibrium pressure and p0 is the saturation pressure of nitrogen at −196 °C.b Determined from powder SAXS measurements shown in Fig. 3. | |||
| SBA-15 | 627 | 1.04 | 10.02 |
| CN1 | 170 | 0.29 | 9.01 |
| CN2 | 191 | 0.31 | 9.73 |
| CN3 | 145 | 0.23 | 7.86 |
| CN4 | 116 | 0.27 | 9.01 |
| Bulk g-C3N4 | 17 | 0.12 | — |
Fig. 4 shows the N2 adsorption–desorption isotherms and corresponding pore size distributions for CNX materials. These curves exhibited a type IV isotherm with a type H1 hysteresis loop, which is the typical characteristic of mesoporous materials, including capillary condensation, as indicated by Fig. 4A. Although the isotherm of bulk g-C3N4 was a type IV one, the type H3 hysteresis loop at high p/p0 reflected the formation of silt-shaped pores caused by aggregates of particles. The Brunauer–Emmett–Teller (BET) specific surface area (Table 1) of bulk g-C3N4 was only 17 m2 g−1, while that of CN2 reached up to 191 m2 g−1, which was the highest value among all the samples. The significant increase in specific surface area can be ascribed to the reservation of the mesostructure of template during the nanocasting process. The pore-size distributions of the samples estimated from the adsorption branches using the Barrett–Joyner–Halenda (BJH) method, as shown in Fig. 4B and S2,† showed that the pore sizes were centered on ∼3.2 nm for CNX and ∼9.2 nm for SBA-15. The pore size of CN2 was only slightly higher than the wall thickness of SBA-15, which probably resulted from the volume shrinkage of the filled DCDA inside the pores during condensation. The high specific surface area and mesopores of ompg-C3N4 were contributed to the great enhancement in photocatalytic activity which would be discussed in the following sections.
 | ||
| Fig. 4 Nitrogen adsorption–desorption isotherms (A) and pore size distributions (B) of various g-C3N4 samples. | ||
TEM (Fig. 5) was further used to study the morphology and microstructure of CN2 along with SBA-15 for the purpose of confirming the successful template replication. Well-distributed cashew-like SBA-15 particles with 0.5–0.7 μm in diameter and 0.9–1.0 μm in length can be observed from the large scale TEM image in Fig. 5A, and a high-magnification TEM image recorded along the (110) direction shown in Fig. 5B revealed that they had highly ordered mesostructures. In the case of CN2, the images in Fig. 5C and D clearly show the same dispersity and mesostructure as SBA-15, which indicated the successful nanocasting modification. The result was consistent with N2 adsorption–desorption and SAXS measurements. As can be shown in Fig. S3A,† bulk g-C3N4, consisting of solid agglomerates with a size of several micrometers, showed that its surface was layered as in the case of graphite. For CN1, Fig. S3B† showed that the cashew-shaped g-C3N4 particles co-existed with some tiny aggregates. The irregular shape was caused by the incomplete filling of the DCDA precursor in the template pores during the nanocasting process. In comparison to CN2, CN3 and CN4 (Fig. S3C and D†) appeared in the form of particle aggregates surrounding wrinkle-like structures. This finding can be ascribed to the high mass ratio of precursor/template; excessive DCDA resulted in agglomeration during the thermal polymerization process. The initial precursor/template weight ratio was inferred to be important for the preparation of ompg-C3N4: when too little (weight ratio: 1) or too much (weight ratio: 3, 4) precursor was used, tiny particles and fibrils (CN1) or irregular aggregates (CN3, CN4) were found in the compound, respectively.
Surface functional groups and composition characterization
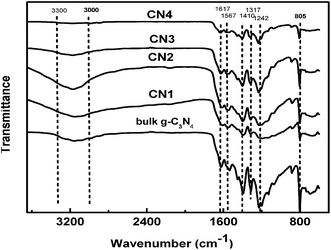
The functional groups of the g-C3N4 samples were characterized by FT-IR spectroscopy and the recorded FT-IR spectra are shown in Fig. 6. It is widely accepted to date that the g-C3N4 is based on tri-s-triazine building blocks.43 In the spectrum of g-C3N4 samples, several absorption bands were found in the region of 1200–1620 cm−1, with peaks at 1242, 1317, 1410, 1567, and 1617 cm−1, which gave strong evidence of the characteristic stretching vibration modes of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C–N heterocycles.44,45 The sharp absorption peak at 801 cm−1 manifested the typical breathing mode of tri-s-triazine units. In addition, the broad bands in the region of 3000–3300 cm−1 can be assigned to the stretching modes of the primary and secondary amines and their intermolecular hydrogen bonding interactions.40 This result indicated that the amino functions still existed in the products even after directly heating the DCDA/SBA-15 composites.
N and C–N heterocycles.44,45 The sharp absorption peak at 801 cm−1 manifested the typical breathing mode of tri-s-triazine units. In addition, the broad bands in the region of 3000–3300 cm−1 can be assigned to the stretching modes of the primary and secondary amines and their intermolecular hydrogen bonding interactions.40 This result indicated that the amino functions still existed in the products even after directly heating the DCDA/SBA-15 composites.
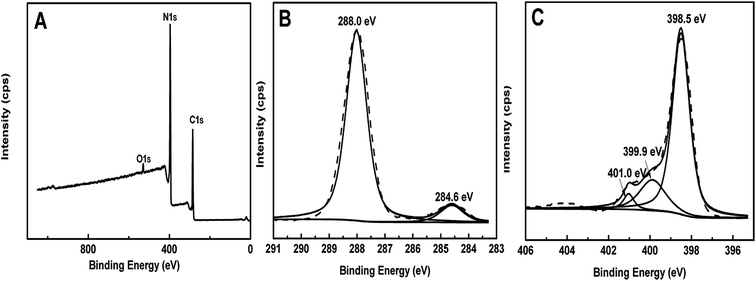
The typical high resolution XPS (Fig. 7) was used to analyze the surface chemical compositions of ompg-C3N4. The binding energies obtained in the XPS analysis were corrected for specimen charging by referencing C 1s to 284.60 eV (extra carbon contamination).46 The survey scan spectrum (Fig. 7A) revealed the presence of C, N, and O (O 1s peak was assigned to the adsorbed H2O on the surface of the photocatalyst47). In addition, Si and F were not found, indicating that the SBA-15 template had been thoroughly etched, and ompg-C3N4 had been well-rinsed. In the C 1s high-resolution XPS (Fig. 7B) spectrum of ompg-C3N4, the major peak at 288.0 eV originated from the coordination between carbon atoms and three N neighbors in its chemical bone structure.46,47 The N 1s XPS (Fig. 7C) spectrum was divided into three component peaks. The major peak at 398.5 eV could be ascribed to the sp2-hybridized N.47 The second peak at 399.9 eV was attributed to –NH2 or ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH groups,47 and the third peak at 401.0 eV was attributed to the charging effects or positive charge localization in heterocycles.48 Based on the XPS spectrum, the surface C/N atomic ratio was calculated to be 0.70
NH groups,47 and the third peak at 401.0 eV was attributed to the charging effects or positive charge localization in heterocycles.48 Based on the XPS spectrum, the surface C/N atomic ratio was calculated to be 0.70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was close to the theoretical value (C/N = 0.75
1, which was close to the theoretical value (C/N = 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in g-C3N4. By combing the XRD, FT-IR and XPS results, we inferred that these CNX materials were mainly built from graphitic sheets with triazine-like CN heterocyclic motifs, as well as a certain amount of uncondensed amino species at the edge of the graphitic sheets. Furthermore, CN2 results were in good agreement with those of other mesoporous CN materials prepared using cyanamide as precursors.33,38,49
1) in g-C3N4. By combing the XRD, FT-IR and XPS results, we inferred that these CNX materials were mainly built from graphitic sheets with triazine-like CN heterocyclic motifs, as well as a certain amount of uncondensed amino species at the edge of the graphitic sheets. Furthermore, CN2 results were in good agreement with those of other mesoporous CN materials prepared using cyanamide as precursors.33,38,49
Optical properties characterization
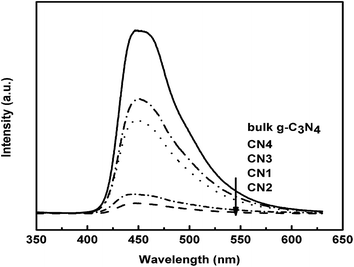
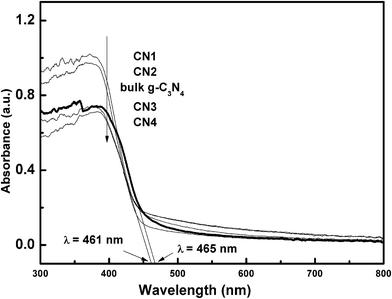
To investigate the “nanocasting” modification effect on the photocatalytic activity of g-C3N4, photoluminescence (PL) spectra analysis (Fig. 8) with excitation wavelength of 325 nm was carried out. It aimed to probe the migration, transfer, and recombination processes of photogenerated electron–hole pairs in g-C3N4-based photocatalysts. The main emission peaks centered around 450 nm for all g-C3N4 samples, similar to the results reported in the literature.16 As we all know, a weaker PL intensity represents a lower recombination probability of electron–hole under light irradiation. The PL intensities of g-C3N4 samples synthesized through the hard template method were far below the intensity of bulk g-C3N4, that is, the recombination rate of photogenerated carriers was greatly inhibited by the modification of nanocasting, indicating that the separation efficiency of the photogenerated electron–hole pairs in CNX was higher than that in bulk g-C3N4. With the optimal precursor/template ratio, the as-synthesized CN2 displayed the lowest PL intensity and possessed the best photocatalytic activity which would be proven in the following degradation experiments.The UV-vis diffuse absorption spectra was employed to characterize the optical absorption properties of the g-C3N4 samples. In Fig. 9, bulk g-C3N4 shows the spectral response from UV to visible region, and the band edge of optical adsorption was around 465 nm, corresponding to the band gap at 2.67 eV. However, all the CNX materials displayed almost the same absorption edge around 461 nm, with a band gap at 2.69 eV which indicates that they absorbed less energy in visible region than the bulk g-C3N4 did. As compared with the bulk g-C3N4, the blue shift of absorption edge of CNX materials might be induced by small size effect caused by the nanocasting modification. Combining with the PL and the following photodegradation results, we inferred that the highly effective separation of photogenerated carriers of CNX played a more important role than their ability to absorb visible light, namely, the effectively separated hole–electron pairs can greatly make up the slightly weak absorption ability in visible light region during the photocatalytic reaction.
It was widely accepted that the separation efficiency of the photogenerated electrons and holes plays a vital role in photocatalytic reaction: the higher photocurrent represents better separation efficiency, thereby resulting in higher photocatalytic activity. Fig. 10 shows the current–time curves for the five samples under several on/off visible-light irradiation cycles. Consistent with the order of PL experimental results, bulk g-C3N4 showed the lowest photocurrent response at 0.05 μA cm−2, while CN2 displayed the highest value at 0.35 μA cm−2 which was 7 and 1.75 times higher than that of bulk g-C3N4 and CN1, respectively. The photocurrent intensity of CN3, at a value of 0.23 μA cm−2, was similar to that of CN1, which was slightly higher than that of CN4. Taking account of the different photocurrent intensities over these five composites especially for CN2 and bulk g-C3N4, we infer that the results could be ascribed to the large amount of reactive sites contained in the mesostructural g-C3N4 photocatalyst.
 | ||
| Fig. 10 Photocurrent responses of bulk g-C3N4, CN1, CN2, CN3, CN4 in 0.5 M Na2SO4 aqueous solution under visible-light irradiation (λ ≥ 420 nm) at 0 V vs. SHE (without bias). | ||
Uniform and reversible photocurrent responses were observed in all electrodes, indicating effective charge transfer and successful electron collection for the five samples within the photoelectrochemical cell. However, the photo-induced current of bulk g-C3N4 (Fig. S4†) was not as stable as that of other g-C3N4 materials synthesized through the nanocasting route. A sharp current peak of bulk g-C3N4 increased at almost the exact moment when the light was switched on, indicating that its degree of crystallinity was not very high. The non-polycondensation of N–H residual bonds, distributed in the graphitic layer structure, generated strong surface states which could serve as electron–hole recombination centers under visible light irradiation,50 hence leading to a slight decrease in the photogenerated current density. Unlike the photocurrent response behavior of bulk g-C3N4 and other CNX materials, CN2 showed a flat, stable and high photocurrent. At this point, nanocasting modification was confirmed to be an efficient way to inhibit the recombination of photo-induced carriers and CN2 was believed to exhibit the best photocatalytic performance.
Enhancement of photocatalytic activity
Seeing that the main absorption peak of MO appears around 464 nm, herein we studied the photocatalytic performance of as-prepared g-C3N4 materials by selecting the visible-light-induced photocatalytic degradation of MO, a typical hazardous dye, as a model reaction. Fig. 11 shows the photocatalytic degradation behavior of MO in the presence of different g-C3N4 photocatalysts under visible light irradiation. At first, adsorption of MO on the g-C3N4 materials must be considered. Fig. 11A shows that adsorption equilibrium was reached within 30 min in the dark for all the g-C3N4 materials, and adsorption amounts of MO on bulk g-C3N4, CN1, CN2, CN3 and CN4 were 2%, 36%, 66%, 54%, and 59%, in that order. Next, each reaction system that was at adsorption equilibrium was irradiated by visible light to allow the photocatalytic process to happen. Because the photocatalytic degradation process occurred on the surface of catalysts, the catalyst with the larger surface area possessed more reactive sites, thus displaying higher catalytic activity. In the case of using bulk g-C3N4 as the photocatalyst (Fig. 11B), the MO concentration only decreased by 12% because of the high recombination of the photoinduced electron–hole pairs. In contrast, other g-C3N4 materials prepared via the nanocasting route exhibited enhanced photocatalytic activity to different degrees, with the lowest degradation efficiency for CN4 being 84% and the highest degradation efficiency for CN2 being nearly 100% under otherwise identical conditions. Fig. S5A† shows that the characteristic absorption peak almost completely disappeared after 48 min of irradiation, further confirming the rapid and complete degradation of MO.Degradation kinetics of MO
Here we tried to put forward a pseudo-first-order reaction model to interpret the experimental data for the photocatalytic degradation of MO| ln(Cad/C) = kapt |
The reasons why the ompg-C3N4 showed better photocatalytic activity than the bulk g-C3N4 and other mesoporous g-C3N4 can be interpreted as follows: firstly, the larger specific surface area enabled the material to adsorb more dye molecules; secondly, the existence of a large amount of the special nanochannels inside the material made the ordered mesoporous g-C3N4 possess more active sites, thereby shortening the diffusion path of free carriers from the bulk to the surface and further suppressing the recombination of photogenerated electron–hole pairs. As a result, the photocatalytic activity of ompg-C3N4 was greatly enhanced.
The stability of photocatalysts is very important from view point of its practical applications. The cycling runs for the photo-oxidation of MO over CN2 catalyst were performed to evaluate its stability under the same photocatalytic conditions. The samples were washed with deionized water and dried after each cycling experiment. Fig. 12A shows the photodegradation of MO in every cycling run, and very slight catalyst deactivation related to mass loss of CN2 was detected with the increasing number of cycles. Therefore, the ompg-C3N4 can be regarded as a stable photocatalyst in visible light photochemical degradation reactions. In addition, the crystalline structure of the CN2 catalyst was also measured by XRD after the five photodegradation runs (Fig. 12B). The XRD pattern of the reused CN2 was similar to that of the freshly prepared catalyst, further demonstrating the stability of the as-prepared ompg-C3N4.
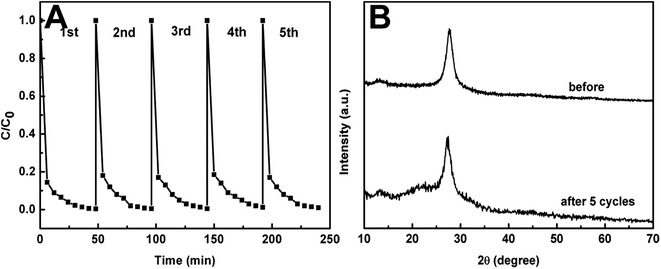 | ||
| Fig. 12 Recycling runs in the photodegradation of MO over CN2 (A), and XRD patterns of CN2 before and after five recycling reactions. | ||
Degradation mechanism of MO
For a common photodegradation reaction, some active species, such as holes (h+), superoxide radicals (˙O2−), and hydroxyl radicals (˙OH) were generated under irradiation of UV-visible light. Generally, h+ can directly react with organic pollutants if the semiconductor photocatalyst has moderate redox potential. The generation of ˙O2− is associated with the photogenerated electron induced direct reduction of O2 (O2 + e− → ˙O2−), whereas ˙OH is formed via the direct hole oxidation (h+ + H2O → ˙OH + H+) or photogenerated electron induced multistep reductions of O2 (O2 + e− → ˙O2−, ˙O2− + e− + 2H+ → H2O2, H2O2 + e− → ˙OH + OH−). In order to ascertain the main reactive species responsible for the degradation of MO, a series of control experiments with quenchers was carried out. Herein, triethanolamine (TEOA) was used to quench h+,51 p-benzoquinone (p-BQ) as ˙O2− scavenger52 and methanol as ˙OH scavenger.53,54 Fig. 13 shows the effects of different scavengers on the degradation activities of CN2. It was seen in Fig. 13A that, the addition of methanol in the system of MO degradation had a minimum effect on the photodegradation activity, which implied that ˙OH was not the active species. The same was true for the addition of TEOA, indicating that ˙OH was also not the main active species in the degradation process. By contrast, a significant suppressing effect on the photodegradation of MO was observed after p-BQ was introduced. In this case, the degradation efficiency of MO was decreased to 49.6%, confirming that the ˙O2− species played a crucial role in the process of photodegradation. The rate constants for MO degradation in the presence of different scavengers were given in Fig. 13B. Obviously, the photocatalytic process was mainly governed by ˙O2−, rather than ˙OH or h+. | ||
| Fig. 13 Effect of different active scavengers on the (A) degradation of MO and (B) the rate constants over CN2. | ||
Based on the detection of the active species in the photodegradation process, and the probable reactions occurring in the photodegradation of MO are described as:
| CN2 (ompg-C3N4) + hν → h+ + e− | (1) |
| O2 + e− → ˙O2− | (2) |
| ˙O2− + MO → degraded mineralized products | (3) |
Conclusions
The ompg-C3N4 materials with excellent photocatalytic performance were synthesized via a green cosolvent-free nanocasting route and applied in visible-light-induced photocatalytic degradation of methyl orange (MO) for the first time. The superior visible-light photocatalytic activity of ompg-C3N4 was attributed to two reasons: (i) the well-dispersed cashew-like ompg-C3N4 greatly enhanced the adsorption ability of dye molecules on the surface of the catalyst due to its large specific surface area; and (ii) the stable mesopores played an important role in the photodegradation process, including shortening the diffusion path of active species and reducing their recombination. The outstanding photocatalytic performance and high stability of ompg-C3N4 make it a promising photocatalyst in efficient utilization of solar energy for the treatment of dye pollutants in environmental pollution control.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21373129, 21176144).References
- A. Houas, H. Lachheb, M. Ksibi, E. Elaloui, C. Guillard and J.-M. Herrmann, Appl. Catal., B, 2001, 31, 145–157 CrossRef CAS.
- C. Ràfols and D. Barceló, J. Chromatogr. A, 1997, 777, 177–192 CrossRef.
- B. Neppolian, H. Choi, S. Sakthivel, B. Arabindoo and V. Murugesan, Chemosphere, 2002, 46, 1173–1181 CrossRef CAS.
- M. Saquib and M. Muneer, Dyes Pigm., 2003, 56, 37–49 CrossRef CAS.
- L. Davydov, E. P. Reddy, P. France and P. G. Smirniotis, Appl. Catal., B, 2001, 32, 95–105 CrossRef CAS.
- C. Hachem, F. Bocquillon, O. Zahraa and M. Bouchy, Dyes Pigm., 2001, 49, 117–125 CrossRef CAS.
- S. Kansal, M. Singh and D. Sud, J. Hazard. Mater., 2007, 141, 581–590 CrossRef CAS PubMed.
- L. G. Devi, S. G. Kumar, K. M. Reddy and C. Munikrishnappa, J. Hazard. Mater., 2009, 164, 459–467 CrossRef PubMed.
- V. K. Gupta, R. Jain, A. Nayak, S. Agarwal and M. Shrivastava, Mater. Sci. Eng C, 2011, 31, 1062–1067 CrossRef CAS PubMed.
- C. Tian, Q. Zhang, A. Wu, M. Jiang, Z. Liang, B. Jiang and H. Fu, Chem. Commun., 2012, 48, 2858–2860 RSC.
- H. Dong, G. Chen, J. Sun, C. Li, Y. Yu and D. Chen, Appl. Catal., B, 2013, 134, 46–54 CrossRef PubMed.
- A. L. Linsebigler, G. Lu and J. T. Yates Jr, Chem. Rev., 1995, 95, 735–758 CrossRef CAS.
- N. Negishi, K. Takeuchi and T. Ibusuki, J. Mater. Sci., 1998, 33, 5789–5794 CrossRef CAS.
- B. O'regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef PubMed.
- J. C. Yu, W. Ho, J. Yu, H. Yip, P. K. Wong and J. Zhao, Environ. Sci. Technol., 2005, 39, 1175–1179 CrossRef CAS.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- J. Zhang, X. Chen, K. Takanabe, K. Maeda, K. Domen, J. D. Epping, X. Fu, S. M. Antonietti and X. Wang, Angew. Chem., Int. Ed., 2010, 49, 441–444 CrossRef CAS PubMed.
- X.-H. Li, X. Wang and M. Antonietti, Chem. Sci., 2012, 3, 2170–2174 RSC.
- H. Shi, G. Chen, C. Zhang and Z. Zou, ACS Catal., 2014, 4, 3637–3643 CrossRef CAS.
- L. Shi, L. Liang, J. Ma, F. Wang and J. Sun, Dalton Trans., 2014, 43, 7236–7244 RSC.
- X. Wang, S. Blechert and M. Antonietti, ACS Catal., 2012, 2, 1596–1606 CrossRef CAS.
- Y. Wang, X. Wang and M. Antonietti, Angew. Chem., Int. Ed., 2012, 51, 68–89 CrossRef CAS PubMed.
- G. Li, D. Zhang and J. C. Yu, Chem. Mater., 2008, 20, 3983–3992 CrossRef CAS.
- L. Shi, L. Liang, F. Wang, M. Liu, S. Zhong and J. Sun, Catal. Commun., 2015, 59, 131–135 CrossRef CAS PubMed.
- M. Zhang, J. Xu, R. Zong and Y. Zhu, Appl. Catal., B, 2014, 147, 229–235 CrossRef CAS PubMed.
- S. C. Lee, H. O. Lintang and L. Yuliati, Chem.–Asian J., 2012, 7, 2139 CrossRef CAS PubMed.
- X. Jin, V. V. Balasubramanian, S. T. Selvan, D. P. Sawant, M. A. Chari, G. Q. Lu and A. Vinu, Angew. Chem., 2009, 48, 7884–7887 CrossRef CAS PubMed.
- S. H. Baeck, K. S. Choi, T. F. Jaramillo, G. D. Stucky and E. W. McFarland, Adv. Mater., 2003, 15, 1269–1273 CrossRef CAS PubMed.
- Y. Zheng, Y. Jiao, J. Chen, J. Liu, J. Liang, A. Du, W. Zhang, Z. Zhu, S. C. Smith, M. Jaroniec, G. Q. Lu and S. Z. Qiao, J. Am. Chem. Soc., 2011, 133, 20116–20119 CrossRef CAS PubMed.
- F. Goettmann, A. Fischer, M. Antonietti and A. Thomas, Angew. Chem., Int. Ed., 2006, 45, 4467–4471 CrossRef CAS PubMed.
- X.-H. Li, J. Zhang, X. Chen, A. Fischer, A. Thomas, M. Antonietti and X. Wang, Chem. Mater., 2011, 23, 4344–4348 CrossRef CAS.
- X. Chen, J. Zhang, X. Fu, M. Antonietti and X. Wang, J. Am. Chem. Soc., 2009, 131, 11658–11659 CrossRef CAS PubMed.
- X. Chen, Y.-S. Jun, K. Takanabe, K. Maeda, K. Domen, X. Fu, M. Antonietti and X. Wang, Chem. Mater., 2009, 21, 4093–4095 CrossRef CAS.
- M. Groenewolt and M. Antonietti, Adv. Mater., 2005, 17, 1789–1792 CrossRef CAS PubMed.
- J. Xu, H.-T. Wu, X. Wang, B. Xue, Y.-X. Li and Y. Cao, Phys. Chem. Chem. Phys., 2013, 15, 4510–4517 RSC.
- X.-X. Zou, G.-D. Li, Y.-N. Wang, J. Zhao, C. Yan, M.-Y. Guo, L. Li and J.-S. Chen, Chem. Commun., 2011, 47, 1066–1068 RSC.
- J. Xu, T. Chen, X. Wang, B. Xue and Y.-X. Li, Catal. Sci. Technol., 2014, 4, 2126–2133 CAS.
- X.-H. Li, X. Wang and M. Antonietti, Chem. Sci., 2012, 3, 2170 RSC.
- C. Yu, J. Fan, B. Tian, D. Zhao and G. D. Stucky, Adv. Mater., 2002, 14, 1742–1745 CrossRef CAS.
- M. J. Bojdys, J. O. Müller, M. Antonietti and A. Thomas, Chem.–Eur. J., 2008, 14, 8177–8182 CrossRef CAS PubMed.
- X. Wang, K. Maeda, X. Chen, K. Takanabe, K. Domen, Y. Hou, X. Fu and M. Antonietti, J. Am. Chem. Soc., 2009, 131, 1680–1681 CrossRef CAS PubMed.
- B. Yuan, Z. Chu, G. Li, Z. Jiang, T. Hu, Q. Wang and C. Wang, J. Mater. Chem. C, 2014, 2, 8212–8215 RSC.
- A. Thomas, A. Fischer, F. Goettmann, M. Antonietti, J.-O. Müller, R. Schlögl and J. M. Carlsson, J. Mater. Chem., 2008, 18, 4893–4908 RSC.
- Q. Xiang, J. Yu and M. Jaroniec, J. Phys. Chem. C, 2011, 115, 7355–7363 CAS.
- Y. Wang, R. Shi, J. Lin and Y. Zhu, Energy Environ. Sci., 2011, 4, 2922–2929 CAS.
- S. Wang, D. Li, C. Sun, S. Yang, Y. Guan and H. He, Appl. Catal., B, 2014, 144, 885–892 CrossRef CAS PubMed.
- S. Martha, A. Nashim and K. Parida, J. Mater. Chem. A, 2013, 1, 7816–7824 CAS.
- Y. Cui, J. Zhang, G. Zhang, J. Huang, P. Liu, M. Antonietti and X. Wang, J. Mater. Chem., 2011, 21, 13032–13039 RSC.
- Y. Zheng, Y. Jiao, J. Chen, J. Liu, J. Liang, A. Du, W. Zhang, Z. Zhu, S. C. Smith, M. Jaroniec, G. Q. Lu and S. Z. Qiao, J. Am. Chem. Soc., 2011, 133, 20116–20119 CrossRef CAS PubMed.
- K. J. McDonald and K.-S. Choi, Chem. Mater., 2011, 23, 4863–4869 CrossRef CAS.
- G. Liao, S. Chen, X. Quan, H. Yu and H. Zhao, J. Mater. Chem., 2012, 22, 2721–2726 RSC.
- E. Baciocchi, T. D. Giacco, F. Elisei, M. F. Gerini, M. Guerra, A. Lapi and P. Liberali, J. Am. Chem. Soc., 2003, 125, 16444–16454 CrossRef CAS PubMed.
- L. Shi, L. Liang, J. Ma and J. Sun, Superlattices Microstruct., 2013, 62, 128–139 CrossRef CAS PubMed.
- R. Dong, B. Tian, J. Zhang, T. Wang, Q. Tao, S. Bao, F. Yang and C. Zeng, Catal. Commun., 2013, 38, 16–20 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional experimental data. See DOI: 10.1039/c5ra13438b |
| This journal is © The Royal Society of Chemistry 2015 |