A core–shell structured magnetic Ag/AgBr@Fe2O3 composite with enhanced photocatalytic activity for organic pollutant degradation and antibacterium
Shuquan Huanga,
Yuanguo Xua,
Zhigang Chena,
Meng Xiea,
Hui Xu*a,
Minqiang He*a,
Huaming Lia and
Qi Zhangb
aSchool of Chemistry and Chemical Engineering, Institute for Energy Research, Jiangsu University, Zhenjiang 212013, P. R. China. E-mail: xh@ujs.edu.cn
bHainan Provincial Key Lab of Fine Chemistry, Hainan University, Haikou, Hainan 570228, P. R. China
First published on 14th August 2015
Abstract
A core–shell structured magnetic Ag/AgBr@Fe2O3 composite was synthesized through a facile solvothermal method. Powder X-ray diffraction (XRD), scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS) and ultraviolet-visible absorption spectroscopy (UV-vis) were applied to characterize the structures and properties of the as-prepared samples. The results indicate that Fe2O3 was coated on the surface of Ag/AgBr and heterostructures were formed. Electrochemistry analysis and photoluminescence (PL) spectra analysis indicate that the introduction of Fe2O3 could improve electron and hole separation efficiency. The photocatalytic activity of the Ag/AgBr@Fe2O3 composites was evaluated by using organic dye methyl orange (MO), endocrine disrupting chemical bisphenol A (BPA) and Escherichia coli (E. coli) as the target pollutants. The as-prepared Ag/AgBr@Fe2O3 composites exhibited much higher photocatalytic activities than pure Ag/AgBr, which was attributed to the effective charge separation of the Ag/AgBr@Fe2O3 composite. In addition, the as-prepared Ag/AgBr@Fe2O3 composite has magnetic properties, therefore after the photocatalytic reaction, it can be quickly separated from solution by an extra magnetic field. Trapping experiments and ESR analysis indicate that the h+ and ˙O2− are the main active species for the photocatalytic degradation. A possible Z-scheme pathway photocatalytic mechanism was proposed.
1. Introduction
In recent years, environmental issues caused by hazardous wastes and water pollution have aroused wide concern. Among these, water pollution which is engendered by chemical pollution and pathogenic microorganisms has been one of the most serious issues in modern society.1 As a promising technology, the semiconductor photocatalysis technique provides a “green” method for environmental purification.2–4 After the discovery of photocatalytic splitting of water on a TiO2 electrode,5 plentiful semiconductor materials have been established.6–13 Among these photocatalysts, plasmonic photocatalysts have been found as an alternative and promising substitute for developing highly efficient visible-light photocatalysts. The plasmonic photocatalysts could dramatically enhance the absorption of visible light and efficiently separate photogenerated electrons and holes because of the surface plasmon resonance (SPR) effect. Generally, noble metal nanoparticles (such as Au and Ag) can photo-induce collective oscillations of the conduction electrons with a resonant frequency, this phenomenon is the surface plasmon resonance effect, which can significantly amplify the absorption of visible light.14 So, the plasmonic photocatalysts provide new opportunities for developing visible light photocatalysts. In recent years, much interest has been paid to the plasmonic photocatalysts based on silver/silver halides (Ag/AgX, X = Cl, Br, I) composite materials due to the surface plasmon resonance of noble metallic Ag.15–21 The silver/silver halides composite photocatalysts have been confirmed that they are not only a photo stable photocatalyst with high visible light photoactivity but also can be used for coupling with other semiconductors to improve the photoactivity. Numerous strategies have been reported for synthesizing Ag/AgX (X = Cl, Br) based photocatalysts, including texturization or morphology control to increase the surface area and band alignment by coupling with other semiconductors. For example, Shen et al.22 reported that Ag/AgCl spherical and sheet like nanoarchitecture morphologies could be controllably fabricated by means of tuning the concentration of cetyltrimethylammonium chloride (CTAC) and AgNO3 aqueous solution. The sheet like Ag/AgCl displayed distinctly boosted catalytic performances toward the photodegradation of methyl orange (MO). Li et al.23 synthesized 1D AgBr@Ag nanostructures by a facile wet chemical method. These AgBr@Ag nanorods have a high surface area and provide more active sites. Many Ag/AgX based composite photocatalysts have been fabricated, such as Ag/AgCl/W18O49, Ag/AgBr/TiO2, and AgCl@Ag@TiO2 etc. These composites not only exhibited very high photocatalytic activity in the photodegradation of organic pollutant but also show very high antibacterial ability under visible light irradiation.24–28 Up to now, this kind of materials has been widely studied in the degradations of non-biodegradable dyes, alcohols, phenols and killing pathogenic bacteria in waste water.29,30 However, the practical applications of these photocatalysts are often limited by the easy loss of the suspended particulate catalysts in the process of photocatalytic reaction and separation. So, finding out an easy way to recover the Ag-based photocatalyst is very necessary. To this end, some strategies have been established, one of them is combining Ag/AgX with a magnetic material. For instance, Tian et al.31 fabricated core–shell structured γ-Fe2O3@SiO2@AgBr:Ag composite microspheres. Dai et al.32 prepared core–shell Ag–AgI/Fe3O4@SiO2 plasmonic photocatalyst. An et al.33 constructed ferromagnetic Fe3O4@SiO2@AgCl:Ag plasmonic nanophotocatalysts, etc. These strategies have achieved great advantages in the development of magnetic plasmonic nanophotocatalysts, but the synthesis route could become cumbersome. Our previous work has attempted to construct ferromagnetic plasmonic nanophotocatalysts by coupling Ag/AgCl with magnetic material CoFe2O4.34 This synthesis route is simple and the photoactivity of Ag/AgCl/CoFe2O4 was enhanced. So, it is necessary to continue investigating high efficient magnetic plasmonic nanophotocatalysts for a long and sustained environment protection strategy.Semiconductor Fe2O3, a kind of magnetic material with band gap 2.0–2.2 eV, can absorb a large fraction of visible light.35 It has lots of advantages in the photocatalytic field, such as environmental-friendly, good chemical stability and low cost.36 Despite these advantages, Fe2O3 suffers from a miserably short excited state lifetime, high recombination rate, poor conductivity and difficulty to separate the photo-induced electron–hole pairs.37–39 Thus pure Fe2O3 may not be a good candidate for photocatalytic reaction. However, as a narrow band gap semiconductor, Fe2O3 could be used for modifying other semiconductors to extend the absorb range for visible light, such as Fe2O3/TiO2,40 Fe2O3/ZnO,41 α-Fe2O3/CdS.42 These results reveal that the introduction of Fe2O3 could enhance their photocatalytic abilities. Furthermore, Fe2O3 has magnetic property, which provides a new sight for fabricating magnetic photocatalysts. Ye et al.43 fabricated magnetically separable polymeric carbon nitride photocatalysts Fe2O3/g-C3N4 and the synergistic effect between Fe2O3 and g-C3N4 enhanced the photocatalytic activity for the degradation of organic dye under visible light. All in all, these results reveal that the Fe2O3 nanoparticles may have potential applications in photocatalysis and magnetic field systems.
Depending on the analysis above, if the hybrid material of Fe2O3 and Ag/AgBr is prepared, it is likely that this hybrid structure with higher photocatalytic performance and magnetic property can be obtained. To the best of our knowledge, there are no existing reports on the preparation of such a core–shell structured Ag/AgBr@Fe2O3 nanoparticle system. In this work, we fabricated magnetic photocatalyst core–shell Ag/AgBr@Fe2O3 composites by a facile solvothermal method. The as-prepared photocatalyst exhibited excellent photocatalytic activity in eliminating methyl orange (MO), bisphenol A (BPA) and Escherichia coli (E. coli) in water under visible light irradiation. This study may provide new insights in the fabrication of high efficient and magnetic plasmonic photocatalysts by a facile method.
2. Experimental
2.1 Materials
All the reagents were analytical grade and used without further purification. They were purchased from Sinopharm Chemical Reagent Co., Ltd.2.2 Preparation of photocatalysts
2.3 Characterization
The X-ray diffraction (XRD) patterns of the crystal phase was collected on a Bruker D8 diffractometer with high-intensity Cu-Kα (λ = 1.5418 Å) in the 2θ range of 10–80°.The SEM images of the samples were obtained on a JEOL-JEM-2010 (JEOL, Japan) operating at 200 kV, which was equipped with an energy-dispersive X-ray spectroscope (EDS) operated at an acceleration voltage of 10 kV. X-ray photoelectron spectroscopy (XPS) was measured on a PHI5300 with a monochromatic Mg Kα source to explore the elements on the surface. The UV-vis diffuse reflectance spectra of the samples were carried out on a Shimadzu UV-2450 UV-vis spectrophotometer (Shimadzu Corporation, Japan) in solid state with the BaSO4 powder was used as the substrate. To diminish the analysis error, a certain amount of samples (about 0.001 g) was mixed with BaSO4 (about 0.009 g) before the UV-vis absorption spectra test. The magnetic properties of the Fe2O3 and Ag/AgBr@Fe2O3 composites were measured in a vibrating sample magnetometer (Quantum Design Corporation, USA) with a maximum applied field of ±2 T. Photocurrent measurements was performed on an electrochemical workstation (CHI 660B, Chenhua Instrument Company, Shanghai, China).2.4 Photocatalytic activity
The photocatalytic activities of the as-prepared samples were assessed by degrading 10 mg L−1 organic dye MO and 10 mg L−1 BPA under 300 W Xe lamp with a UV cutoff filter (λ > 420 nm). And all photocatalytic experiments were performed at 30 °C by using a circulating water system to prevent thermal catalytic effects. In addition, an air pump was used to ensure a constant supply of oxygen during the photoreactions. In a typical procedure, 0.07 g as-prepared samples were dispersed in 70 mL above MO or BPA solution. The mixture was magnetically stirred for about 30 min in the dark to ensure the saturated adsorption of organic pollutant on the surface of catalysts. At regular intervals, approximately 4 mL of the suspension was extracted and then centrifuged to remove essentially all the catalysts and the remaining liquid were collected for further test. The remaining MO degradations were analyzed by a UV-vis spectrophotometer (UV-2450, Shimadzu) at wavelength 463 nm. The remaining BPA degradations were analyzed by the two Varian ProStar 210 pumps, an Agilent TC-C18 column, and a Varian ProStar 325 UV-vis Detector at 230 nm. The mobile phase was 1 mL min−1 with a solution of methanol and H2O with the volume ratio 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 and 20 μL of the sample solution was injected.
25 and 20 μL of the sample solution was injected.
2.5 Antibacterial activity
Prior to experiment, all the glassware and the culture medium solution were sterilized by autoclaving at 121 °C for 20 min. And all the experiments were processed under sterile conditions. The antibacterial activities of Ag/AgBr@Fe2O3 composites were evaluated via the inactivation of Escherichia coli (E. coli) bacteria. The zone of inhibition test was carried out to identify the antibacterial activities of the prepared photocatalysts in dark. Powder photocatalysts were placed on the nutrient agar medium which has been inoculated with 20 μL of the prepared Escherichia coli (E. coli) bacteria suspension. Then the plates were incubated at 37 °C for 16 h in dark.Photocatalytic disinfection experiments were also performed. In a typical procedure, 10 mg of the as-prepared sample was suspended in 20 mL culture medium solution which contains 1/(2 × 104) concentration of the original E. coli concentration. Then the mixture was magnetically stirred in the dark for 10 min, subsequently, the light was switched on to start irradiation. 20 μL of the solution was sampled at the time intervals of 0 min, 5 min, 10 min and 13 min. Each solution was dispersed on the nutrient agar medium and incubated at 37 °C for 16 h in dark.
3. Results and discussion
3.1 XRD analysis
The X-ray diffraction (XRD) pattern of AgBr, Fe2O3 and Ag/AgBr@Fe2O3 composites are shown in Fig. 1A. In the patterns of AgBr (Fig. 1a), it is obvious that all the diffraction peak at 2θ = 26.7°, 31.0°, 44.3°, 52.5°, 55.0°, 64.5°and 73.2° (marked with “♠”) appeared, which matched exactly with the (1 1 1), (2 0 0), (2 2 0), (3 1 1), (2 2 2), (4 0 0) and (4 2 0) crystal planes of cubic phase AgBr (JCPDS No. 06-0438).44 For the pattern of Fe2O3 (Fig. 1f), the diffraction peaks at 2θ = 24.2°, 33.2°, 35.6°, 40.9°, 43.3°, 49.5°, 54.1°, 57.6°, 62.5° and 64.1° (marked with “♦”) are assigned to the (012), (104), (110), (113), (202), (024), (116), (018), (214) and (300) crystal planes of hematite structure α-Fe2O3 (JCPDS No. 33-0664).45 These peaks at 30.3°, 35.6°, 43.3° and 63.1° (marked with “♣”) cannot be ignored in this pattern, and they could be indexed to the (220), (311), (400) and (440) crystal planes of cubic phase γ-Fe2O3 (JCPDS No. 39-1346).46 This result suggests that the as-prepared Fe2O3 contains both hematite structure α-Fe2O3 and cubic phase γ-Fe2O3. The strong magnetic property of the as-prepared Fe2O3 is due to the γ-Fe2O3 phase (maghemite). It is difficult to find the peaks of Fe2O3 in the patterns of Ag/AgBr@Fe2O3 composites, when the mass fraction of Fe2O3 is less than 15%. There are two possible reasons. The first one is that the peaks of Fe2O3 are covered because the peaks of AgBr are very strong. The second one is that the Fe2O3 loaded on the surface of AgBr and were well dispersed. Similar result can also be found in other systems.47 However, we magnified the patterns of 1% Ag/AgBr@Fe2O3, 3% Ag/AgBr@Fe2O3 and 5% Ag/AgBr@Fe2O3 samples by narrow-scan diffraction patterns, as showed in Fig. 1B. The peaks of Fe2O3 still could not be noticed. So, we believe the later is the main reason and this conjecture has also been confirmed by the followed SEM, EDS and XPS analyses.3.2 SEM and EDS analysis
Fig. 2 shows the morphology of Fe2O3, Ag/AgBr and the dispersion state of Fe2O3 on the surface of Ag/AgBr, which were examined by SEM. Fig. 2a and b are the morphology image of the pure Fe2O3, it can be seen that the Fe2O3 particles are granular like with diameters of ca. 20–50 nm. Fig. 2c shows the morphology image of the pure Ag/AgBr, the large particles with diameters of ca. 7–15 μm are AgBr particles and some of the small particles attached on their surface are Ag NPs.48 By close observation (Fig. 2d), these Ag NPs are scattering on the surface of AgBr sparsely. This may be the reason why the peaks of Ag0 couldn't be found in the XRD patterns. Fig. 2e and f are low and high magnification SEM images of 1% Ag/AgBr@Fe2O3 composites, respectively. In the low magnification SEM image, it can be seen that the introduction of Fe2O3 didn't change the shape of Ag/AgBr largely, while in high magnification SEM image (Fig. 2f), it can be clearly seen that Fe2O3 NPs were highly distributed throughout the surface of Ag/AgBr. Similarly, the SEM images of 3% Ag/AgBr@Fe2O3 and 5% Ag/AgBr@Fe2O3 are shown in Fig. 2g, h and i, j, respectively. It can be seen that with the increase of Fe2O3 content more and more holes can be found on the surface of Ag/AgBr, indicating that some of the Fe2O3 are wrapped inside of AgBr particles. Similar results can also be found in our previous work.49 In addition, it can be noticed that an agglomeration of Fe2O3 particle was formed when the content of Fe2O3 in a high level (>3%), this may decreases the photocatalytic activity of Ag/AgBr@Fe2O3 composites. At the same time, EDS of the pure Ag/AgBr and 3% Ag/AgBr@Fe2O3 have also been taken out and shown in Fig. 2k and l, respectively. Only Ag and Br can be found in the image of Ag/AgBr, while in the image of 3% Ag/AgBr@Fe2O3 composites Ag, Br, Fe and O were directly observed. Combining the results of XRD, SEM and EDS, it can be confirmed that Ag/AgBr@Fe2O3 has been successfully synthesized.3.3 XPS analysis
XPS analysis was carried out to further determine the elemental compositions and chemical status of the as-prepared Ag/AgBr and Ag/AgBr@Fe2O3 composite. The results were shown in Fig. 3. The full scan survey XPS spectrum of the Ag/AgBr and 3% Ag/AgBr@Fe2O3 composite indicate that the product consists of Ag, Br, Fe, O and C elements (Fig. 3a). The C element is from the XPS instrument itself. Fig. 3b shows the high-resolution XPS spectrum of Ag 3d, it consists two individual peaks at about 366.00–367.00 eV and 372.00–373.00 eV, which are attributed to Ag 3d5/2 and Ag 3d3/2 binding energies, respectively.50 In contrast to Ag/AgBr, the Ag 3d spectra of Ag/AgBr@Fe2O3 composite show a little shift, and the decrease in the binding energy with an amount of ∼0.4 eV might be attributed to the interaction of Ag+ ions of the composite with the Fe2O3 nanoparticles. It also suggests that the interaction between Fe2O3 and Ag/AgBr existed and the combination sites are Ag elements.51,80 The spectra of Br 3d is shown in Fig. 3c. The binding energies of Br 3d5/2 and Br 3d3/2 are about 67.69 eV and 68.58 eV, respectively, which can be attributed to the Br atoms existing in a Br− state.52 In addition, it is worth noting that both the intensity of Ag 3d and Br 3d of Ag/AgBr@Fe2O3 composite are much weaker than that of Ag/AgBr, respectively. Considering that the main components of the composites are Ag/AgBr, this result confirmed that the Fe2O3 NPs were covered on the surface of Ag/AgBr again. Fig. 3d shows the spectra of Fe 2p, the two peaks at about 710.54 eV and 724.15 eV are attributed to the binding energies of Fe 2p3/2 and Fe 2p1/2, respectively. The high-resolution O 1s XPS spectra of Ag/AgBr and Ag/AgBr@Fe2O3 samples are shown in Fig. 3e. The peak at about 529.71 eV can be assigned to the oxygen in Fe2O3 crystals,53 and the peak at about 531.52 eV is considered to be the enthetic O, such as ambient adsorption.54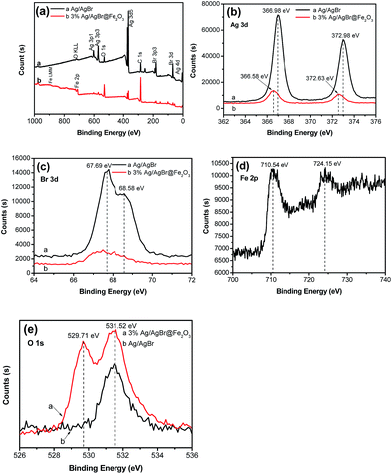 | ||
| Fig. 3 XPS spectra of the pure Ag/AgBr and 3% Ag/AgBr@Fe2O3 composite. (a) Survey of the sample; (b) Ag 3d; (c) Br 3d; (d) Fe 2p and (e) O 1s. | ||
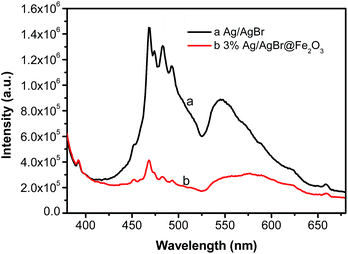
3.4 UV-vis absorption spectra analysis
Fig. 4 shows the UV-vis absorption spectra (in the diffuse reflectance spectra mode) and corresponding Tauc's plots of (αhv)2 vs. (hv) of as-prepared samples.55 From the UV-vis absorption spectra (Fig. 4a), it can be seen that the bare Ag/AgBr has a main absorption region from 200 to 450 nm. And the absorption in the range 500–700 nm is ascribed to the SPR effect of Ag nanoparticles on the surface of the AgBr, which indicates the existence of Ag0 in the composite catalysts. This result is in accordance with our previous work.48 For the Ag/AgBr@Fe2O3 composites, an apparent absorption enhancement for light is observed compared to the pure Ag/AgBr, which is thought to result from Ag/AgBr covered by Fe2O3. This result is consistent with the above SEM and XPS analyses. Furthermore, the light absorption ability was enhanced with the increased Fe2O3 contents. The enhanced light absorption may lead to forming more electron–hole pairs.56 In addition, it shouldn't be neglected that the light absorption ability of pure Fe2O3 is less than that of Ag/AgBr@Fe2O3 composites in the range 600–800 nm, which suggests a synergistic effect between Ag/AgBr and Fe2O3.57 The band gap energy Eg of the as-prepared Fe2O3 and Ag/AgBr was estimated from the corresponding Tauc's plots of (αhv)2 vs. (hv),54 and the result is shown in the Fig. 4b. The band gap energy of as-prepared Fe2O3 and Ag/AgBr was estimated to be 2.1 eV and 2.6 eV, respectively, which is similar to the reported literatures.51,58 In addition, to study the flowchart of photo-excited charge carriers, potentials of the conduction band (CB) and valence band (VB) edges of as-prepared Fe2O3 was evaluated by Mulliken electronegativity theory: ECB = X − EC − 0.5(Eg), where X was the absolute electronegativity of the atom semiconductor, EC was the energy of free electrons with the hydrogen scale (4.5 eV); Eg was the band gap of the semiconductor. Therefore, the conduction band (CB) of Fe2O3 and AgBr was determined to be 0.3 eV and 0 eV, respectively, which are similar to the reported literatures.59,60 Then the valence band (VB) can be determined by EVB = ECB + Eg,61 and the values are 2.4 eV and 2.6 eV for Fe2O3 and AgBr, respectively.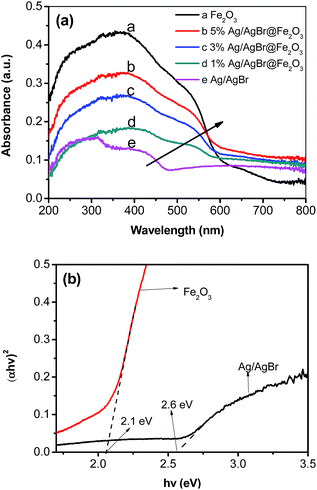 | ||
| Fig. 4 (a) UV-vis absorption spectra of as-prepared samples and (b) corresponding Tauc's plots of (αhv)2 vs. (hv) of Fe2O3 and Ag/AgBr. | ||
3.5 Electrochemistry analysis
It is widely accepted that the higher photocurrent was, the better electron and hole separation efficiency would be.62,63 Fig. 5 shows 5 on–off cycles visible light irradiation transient photocurrent responses of Ag/AgBr and 3% Ag/AgBr@Fe2O3 hybrid material. Obviously, the 3% Ag/AgBr@Fe2O3 hybrid material have higher photocurrent response than that of the Ag/AgBr. It indicates that the 3% Ag/AgBr@Fe2O3 hybrid material has a higher separation rate of photoexcited electrons and holes under the irradiation of visible light.64,65 In addition, it can be seen that the photocurrent value rapidly decreased and increased to a constant value when the light was off and on. This result demonstrated that the recombination of electrons and holes was inhibited greatly, which is good for photocatalytic reaction.663.6 PL analysis
To further confirm that the introduction of Fe2O3 could reduce the electrons and holes recombination rate. PL spectra for Ag/AgBr and 3% Ag/AgBr@Fe2O3 hybrid material were presented in Fig. 6. It is obvious that PL intensity of 3% Ag/AgBr@Fe2O3 decreased significantly compared to that of Ag/AgBr, which means efficient transfer of photoexcited electrons between Fe2O3 and Ag/AgBr. This result indicates that the introduction of Fe2O3 favors the effective charge separation of Ag/AgBr and improve the photocatalytic activities.673.7 VSM analysis
Fig. 7 shows the magnetic behavior of as-prepared Fe2O3 and 3% Ag/AgBr@Fe2O3 composite, which is investigated by a vibrating sample magnetometer at room temperature. The curve presents almost no hysteresis loop, which suggests that the as-prepared Fe2O3 and 3% Ag/AgBr@Fe2O3 composite have ferromagnetic behavior.68 The magnetization saturation (Ms) values are 34.9 and 3.3 emu g−1 for Fe2O3 and 3% Ag/AgBr@Fe2O3 composite, respectively. The magnetic separation ability of the 3% Ag/AgBr@Fe2O3 composite was tested in water by placing a magnet near the glass bottle. The red particles were attracted toward the magnet within a short time (Fig. 6 inset graph). Therefore, this will provide an easy and efficient way to separate the photocatalysts from water under an external magnetic field. | ||
| Fig. 7 The hysteresis loops of pure Fe2O3 and 3% Ag/AgBr@Fe2O3 composite (the inset graph shows the sample dispersed in water (left) and separated by external magnet (right)). | ||
3.8 Photocatalytic activity
Photodegradation of MO under visible light irradiation was carried out to evaluate the photocatalytic ability of the as-prepared samples. As shown in Fig. 8a, it can be seen that MO self-decomposition is negligible. The pure Ag/AgBr could degrade MO by 55.1% after 12 min under visible light irradiation. The Ag/AgBr@Fe2O3 composites show much better photocatalytic activity than Ag/AgBr alone. After visible light irradiation for 12 min, the degradation efficiency of MO over 1% Ag/AgBr@Fe2O3, 3% Ag/AgBr@Fe2O3 and 5% Ag/AgBr@Fe2O3 are 91.7%, 94.4% and 82.7%, respectively. It can be noticed that the photocatalytic ability of Ag/AgBr@Fe2O3 composites is greatly influenced by the content of Fe2O3. When the Fe2O3 content is 3%, it has the best photocatalytic activity. Whatever the Fe2O3 content is high or less than 3% (such as 1% and 5%), its photocatalytic activity decreased. The linear relationship of −ln(C/C0) versus time is shown in Fig. 8b and the pseudo-first-order constants and relative coefficients are summarized in Table 1. The results indicate that the photocatalytic activity was improved by the introduction of Fe2O3 compared to pure Ag/AgBr. Especially, the 3% Ag/AgBr@Fe2O3 composite exhibit about 3.81 times degradation rate as high as that of pure Ag/AgBr. Fig. 8c shows the time-dependent absorption spectra of MO solution in the presence of 3% Ag/AgBr@Fe2O3 composite. It can be seen that the color of MO solution changed from yellow to light yellow and then colourless during the reaction (Fig. 8c inset graph). An evident decrease in MO absorption at λ = 463 nm was observed, indicating that the chromophoric structure was destroyed.| Sample | Kinetic constant (k, min−1) | R2 |
|---|---|---|
| Ag/AgBr | 0.06078 | 0.98979 |
| 1% Ag/AgBr@Fe2O3 | 0.13290 | 0.98047 |
| 5% Ag/AgBr@Fe2O3 | 0.19153 | 0.98513 |
| 3% Ag/AgBr@Fe2O3 | 0.23217 | 0.98847 |
Bisphenol-A (2,2-bis(4-hydroxyphenyl)propane, BPA), which is a compounds termed endocrine disruptor, has frequently been detected in surface water due to it is widely used in the production of polycarbonate plastics and epoxy resins. The toxicity tests revealed that it may cause various adverse effects on aquatic organisms even at low exposure levels.69 Therefore, the degradation of BPA is important. In this work, the degradation of BPA under visible light with the as-prepared samples also has been studied. As shown in Fig. 9a, it can be seen that the BPA couldn't self-decomposition without catalysts. The pure Ag/AgBr has very low degradation efficiency for BPA, and only 26.0% BPA were degraded within 3 h. While the Ag/AgBr@Fe2O3 composites exhibit a relative higher photodegradation for BPA, as same as the photodegradation for MO, the 3% Ag/AgBr@Fe2O3 composite exhibit the best photocatalytic activity, it can degrade BPA up to 69.0% within 3 h. The HPLC analysis of BPA solution in the presence of 3% Ag/AgBr@Fe2O3 composite has been taken out. The results showed in Fig. 9b, and it is obvious that the characteristic peak of BPA decreased with the photoreaction process, which confirmed that BPA was indeed degraded by 3% Ag/AgBr@Fe2O3 composite.
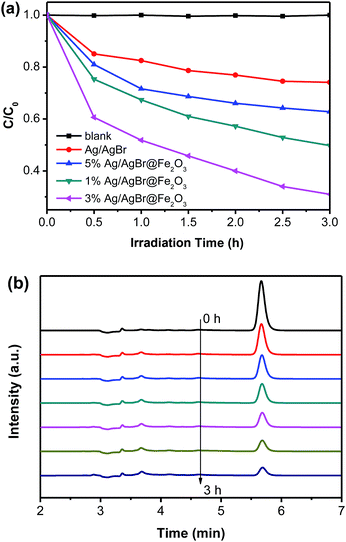 | ||
| Fig. 9 (a) Photodegradation of BPA by as-prepared samples. (b) The HPLC of the BPA degraded solution for different times in the presence of 3% Ag/AgBr@Fe2O3. | ||
3.9 Photocatalytic antibacterial activity
Fig. 10a shows the results of the antibacterial experiment of 3% Ag/AgBr@Fe2O3 in the dark. It is obvious that the 3% Ag/AgBr@Fe2O3 composite has a clear inhibition zone of about 16 mm against E. coli. This result indicates 3% Ag/AgBr@Fe2O3 has a good antibacterial ability in the dark. Fig. 10b shows the results of the photocatalytic antibacterial experiments. It can be seen that the amount of E. coli was not obviously decrease under the visible light irradiation, indicating that the E. coli could survive under visible light irradiation. In the presence of 3% Ag/AgBr@Fe2O3 composite, numerous E. coli were still alive without light irradiation. However, when the system was irradiated by visible light for 5 min, more than half of the E. coli were killed. When the system was irradiated for 10 min, only a small number of E. coli were survived. When the system was irradiated for 13 min all the E. coli were killed. This result indicates that 3% Ag/AgBr@Fe2O3 possesses antibacterial ability under visible light irradiation.3.10 Cycling runs
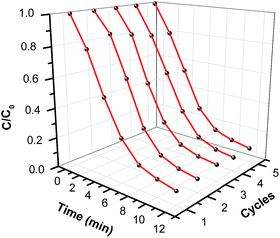
The recycle ability of magnetic photocatalyst is an important property for practical applications. To evaluate the reusability of the Ag/AgBr@Fe2O3 hybrid material, recycling reactions for degrading MO over 3% Ag/AgBr@Fe2O3 under visible light were carried out. As shown in Fig. 11, the MO still can be degraded up to 89.1% after five consecutive cycles, which implies the high recycle ability of Ag/AgBr@Fe2O3 hybrid material.3.11 Mechanism of pollutant photodegradation
To study the photodegradation mechanism, the trapping experiments of active species were taken out by using 2-propanol as the hydroxyl radical (˙OH) scavenger, N2-bubbling for inhibiting the superoxide radical (˙O2−), and triethanolamine (TEOA) as the hole (h+) radical scavenger.70 The results are shown in Fig. 12. It can be seen that the degradation efficiency of MO was hardly affected by adding 1 mM 2-propanol, indicating that the ˙OH is not the main reactive species. The degradation rate of MO was decreased under an N2 atmosphere, suggesting that the ˙O2− plays an important role in photodegradation process. When 1 mM TEOA was added, the degradation rate of MO was greatly inhibited, which reveals that the h+ was a dominant reactive species. To further confirm the active species in the photodegradation process, the electron spin resonance (ESR) spin-trap technique were carried out. The Ag/AgBr and 3% Ag/AgBr@Fe2O3 composite were dispersed with DMPO in water and irradiated for 8 min by a Quanta-Ray Nd:YAG pulsed laser system, and the results are shown in Fig. 13. Under visible light irradiation, the characteristic signals of both the DMPO-superoxide radical (˙O2−) and the DMPO-hydroxyl radical (˙OH) could be observed. In addition, it is obviously that the signal intensity of the 3% Ag/AgBr@Fe2O3 is much higher than that of Ag/AgBr for both the DMPO-superoxide radical (˙O2−) and the DMPO-hydroxyl radical (˙OH). This result reveals a synergistic effect between AgBr and Fe2O3, which could enhance the formation of reactive species thus improve photocatalytic performance. The ESR analysis indicate that the ˙O2− and ˙OH are formed, and this result is a little different from the trapping experiments result. Both ESR analysis and trapping experiments revealed ˙O2− plays an important role in photodegradation process, but it is very different for ˙OH. Trapping experiments revealed ˙OH did not obvious affect the degradation rate of MO, however, ESR analysis revealed the characteristic signals of DMPO-hydroxyl radical (˙OH). It is well known that the ˙OH could oxide MO in solution.71,72 So, if the ˙OH is actually formed in the MO degradation process, the trapping experiment of ˙OH will reveal the according result, but in fact it didn't. Since the ˙OH mainly come from oxidizing OH− or H2O by the photogenerated holes. Considering that the ESR experiments and the trapping experiments were performed under different solutions, suggesting a very little amount of ˙OH were formed in the MO degradation process due to the holes have directly oxidized MO. But the ˙OH will formed in the ESR experiment. It is well known that photocatalyst adsorption ability for dye also play a critical role in the photo-degradation process. Fig. 14 shows the comparison of MO adsorption ability of pure Ag/AgBr and 3% Ag/AgBr@Fe2O3 composite in 15 min. It can be seen that at a same origin MO concentration, the MO adsorption ability of 3% Ag/AgBr@Fe2O3 composite is much higher than that of pure Ag/AgBr. Therefore, an adsorption oxidation mechanism was proposed, as discussed below.Based on the results of above researches, a possible Z-scheme pathway for the adsorption and photocatalytic degradation of MO over Ag/AgBr@Fe2O3 was proposed, as shown in Fig. 15. A uniquely hierarchical nanostructure was formed when the Fe2O3 nanocrystals were introduced on the surfaces of Ag/AgBr, which would provide a high surface area and numerous active sites for the photocatalytic degradation of organic substances. Under visible light, both Fe2O3 and Ag/AgBr were excited. Metallic Ag nanoparticles can absorb visible light, and generated electron–hole pairs.73 On the one hand, the electrons will transfer to the CB of AgBr or are captured by the O2 in the solution to form ˙O2−.74–78 On the other hand, the remained holes on the metallic Ag nanoparticles will recombine with the electrons that has been excited on the CB of Fe2O3 due to the high Schottky barrier at the metal–semiconductor interface.79 For the VB holes, since the VB of AgBr is 2.6 eV, which is higher than that of the Fe2O3 (2.4 eV). So, the holes on the VB of AgBr will transfer to the VB of Fe2O3 and directly oxidize organic substances that have been adsorbed in the layer of Fe2O3, but neither oxidize OH− nor H2O into ˙OH. In sum, the Fe2O3 on the surface of the photocatalysts could improve the adsorbability for organic substances and the interaction between Fe2O3, metallic Ag nanoparticles and AgBr greatly improved the separation efficiency of electron–hole pairs and thus enhanced the photocatalytic performance.
4. Conclusions
In summary, core–shell structured Ag/AgBr@Fe2O3 magnetic photocatalyst was prepared successfully via a facile solvothermal process. The Ag/AgBr were covered by Fe2O3 and formed a uniquely core–shell nanostructure, which would provide a high surface area and numerous active sites for the photocatalytic reaction. After the introduction of Fe2O3, the Ag/AgBr@Fe2O3 composites exhibit increased photocatalytic activity in the degradation of MO and BPA. The 3% Ag/AgBr@Fe2O3 composite exhibited the optimal photocatalytic performance. This enhancement could be attributed to the unique CB positions of Fe2O3 and Ag/AgBr result in a Z-scheme pathway photocatalytic mechanism, which could improve the separation efficiency of electron–hole pairs. Furthermore, the Ag/AgBr@Fe2O3 composite has antibacterial and magnetic ability, which have greatly extended the applications of Ag/AgBr based photocatalysts.Acknowledgements
This work is financially supported by the National Natural Science Foundation of China for Youths (No. 21407065), Natural Science Foundation of Jiangsu Province for Youths (BK20140533, BK2012717), China Postdoctoral Science Foundation (No. 2014M551520, 2014M560399), Jiangsu Postdoctoral Science Foundation (1401143C) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.Notes and references
- R. Kumar, S. Anandan, K. Hembram and T. N. Rao, ACS Appl. Mater. Interfaces, 2014, 6, 13138–13148 CAS.
- C. C. Chen, W. H. Ma and J. C. Zhao, Chem. Soc. Rev., 2010, 39, 4206–4219 RSC.
- A. L. Linsebigler, G. Q. Lu and J. T. Yates, Chem. Rev., 1995, 95, 735–758 CrossRef CAS.
- L. S. Zhang, K. H. Wong, Z. Chen, J. C. Yu, J. C. Zhao, C. Hu, C. Y. Chan and P. K. Wong, Appl. Catal., A, 2009, 363, 221–229 CrossRef CAS PubMed.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253–278 RSC.
- H. Kato, K. Asakura and A. Kudo, J. Am. Chem. Soc., 2003, 125, 3082–3089 CrossRef CAS PubMed.
- Z. G. Zou, J. H. Ye, K. Sayama and H. Arakawa, Nature, 2001, 414, 625–627 CrossRef CAS PubMed.
- K. Maeda, K. Teramura, D. L. Lu, T. Takata, N. Saito, Y. Inoue and K. Domen, Nature, 2006, 440, 295 CrossRef CAS PubMed.
- Z. G. Yi, J. H. Ye, N. Kikugawa, T. Kako, S. X. Ouyang, H. Stuart-Williams, H. Yang, J. Y. Cao, W. J. Luo, Z. S. Li, Y. Liu and R. L. Withers, Nat. Mater., 2010, 9, 559–564 CrossRef CAS PubMed.
- X. B. Chen, S. H. Shen, L. J. Guo and S. S. Mao, Chem. Rev., 2010, 110, 6503–6570 CrossRef CAS PubMed.
- X. B. Chen, L. Liu, P. Y. Yu and S. S. Mao, Science, 2011, 331, 746–750 CrossRef CAS PubMed.
- F. E. Osterloh, Chem. Mater., 2008, 20, 35–54 CrossRef CAS.
- L. Q. Ye, J. Y. Liu, C. Q. Gong, L. H. Tian, T. Y. Peng and L. Zan, ACS Catal., 2012, 2, 1677–1683 CrossRef CAS.
- P. Wang, B. B. Huang, X. Y. Qin, X. Y. Zhang, Y. Dai, J. Y. Wei and M. H. Whangbo, Angew. Chem., Int. Ed., 2008, 47, 7931–7933 CrossRef CAS PubMed.
- P. Wang, B. B. Huang, Z. Lou, X. Zhang, X. Qin, Y. Dai, Z. Zheng and X. Wang, Chem.–Eur. J., 2010, 16, 538–544 CrossRef CAS PubMed.
- P. Wang, B. B. Huang, X. Y. Zhang, X. Y. Qin, H. Jin, Y. Dai, Z. Y. Wang, J. Y. Wei, J. Zhan, S. Y. Wang, J. P. Wang and M. H. Whangbo, Chem.–Eur. J., 2009, 15, 1821–1824 CrossRef CAS PubMed.
- C. An, S. Peng and Y. Sun, Adv. Mater., 2010, 22, 2570–2574 CrossRef CAS PubMed.
- M. S. Zhu, P. L. Chen and M. H. Liu, ACS Nano, 2011, 5, 4529–4536 CrossRef CAS PubMed.
- H. Xu, H. Li, J. Xia, S. Yin, Z. Luo, L. Liu and L. Xu, ACS Appl. Mater. Interfaces, 2011, 3, 22–29 CAS.
- Y. Y. Li and Y. Ding, J. Phys. Chem. C, 2010, 114, 3175–3179 CAS.
- Y. F. Shen, P. L. Chen, D. Xiao, C. C. Chen, M. S. Zhu, T. S. Li, W. G. Ma and M. H. Liu, Langmuir, 2015, 31, 602–610 CrossRef CAS PubMed.
- B. Li, H. Wang, B. W. Zhang, P. F. Hu, C. Chen and L. Guo, ACS Appl. Mater. Interfaces, 2013, 5, 12283–12287 CAS.
- H. X. Shi, G. S. Li, H. W. Sun, T. C. An, H. J. Zhao and P. K. Wong, Appl. Catal., B, 2014, 158, 301–307 CrossRef PubMed.
- H. Tong, S. X. Ouyang, Y. P. Bi, N. Umezawa, M. Oshikiri and J. H. Ye, Adv. Mater., 2012, 24, 229–251 CrossRef CAS PubMed.
- W. B. Hou and S. B. Cronin, Adv. Funct. Mater., 2013, 23, 1612–1619 CrossRef CAS PubMed.
- Z. Z. Lou, Z. Y. Wang, B. B. Huang and Y. Dai, ChemCatChem, 2014, 6, 2456–2476 CrossRef CAS PubMed.
- B. Z. Tian, R. F. Dong, J. M. Zhang, S. Y. Bao, F. Yang and J. L. Zhang, Appl. Catal., B, 2014, 158, 76–84 CrossRef PubMed.
- P. Wang, B. B. Huang, Y. Dai and M. H. Whangbo, Phys. Chem. Chem. Phys., 2012, 14, 9813–9825 RSC.
- B. Z. Tian and J. L. Zhang, Catal. Surv. Asia, 2012, 16, 210–230 CrossRef CAS.
- B. Z. Tian, T. T. Wang, R. F. Dong, S. Y. Bao, F. Yang and J. L. Zhang, Appl. Catal., B, 2014, 147, 22–28 CrossRef CAS PubMed.
- J. F. Guo, B. W. Ma, A. Y. Yin, K. N. Fan and W. L. Dai, Appl. Catal., B, 2011, 101, 580–586 CrossRef CAS PubMed.
- C. H. An, X. J. Ming, J. Z. Wang and S. T. Wang, J. Mater. Chem., 2012, 22, 5171–5176 RSC.
- Y. G. Xu, T. Zhou, S. Q. Huang, M. Xie, H. P. Li, H. Xu, J. X. Xia and H. M. Li, RSC Adv., 2015, 5, 41475–41483 RSC.
- S. Yang, Y. Y. Xu, Y. Q. Sun, G. Y. Zhang and D. Z. Gao, CrystEngComm, 2012, 14, 7915–7921 RSC.
- X. S. Zhang, H. C. Li, S. J. Wang, F. R. F. Fan and A. J. Bard, J. Phys. Chem. C, 2014, 118, 16842–16850 CAS.
- Y. J. Lin, S. Zhou, S. W. Sheehan and D. W. Wang, J. Am. Chem. Soc., 2011, 133, 2398–2401 CrossRef CAS PubMed.
- B. Klahr, S. Gimenez, F. F. Santiago, T. Hamann and J. Bisquert, J. Am. Chem. Soc., 2012, 134, 4294–4302 CrossRef CAS PubMed.
- J. H. Kennedy and K. W. Frese, J. Electrochem. Soc., 1978, 125, 709–714 CrossRef CAS PubMed.
- L. L. Peng, T. F. Xie, Y. C. Lu, H. M. Fan and D. J. Wang, Phys. Chem. Chem. Phys., 2010, 12, 8033–8041 RSC.
- W. Wu, S. F. Zhang, X. H. Xiao, J. Zhou, F. Ren, L. L. Sun and C. Z. Jiang, ACS Appl. Mater. Interfaces, 2012, 4, 3602–3609 CAS.
- Y. Shi, H. Y. Li, L. Wang, W. Shen and H. Z. Chen, ACS Appl. Mater. Interfaces, 2012, 4, 4800–4806 CAS.
- S. Ye, L. G. Qiu, Y. P. Yuan, Y. J. Zhu, J. Xia and J. F. Zhu, J. Mater. Chem. A, 2013, 1, 3008–3015 CAS.
- J. Wang, C. An, J. Liu, G. Xi, W. Jiang, S. Wang and Q. H. Zhang, J. Mater. Chem. A, 2013, 1, 2827–2832 CAS.
- Y. H. Yan, H. Y. Guan, S. Liu and R. Y. Jiang, Ceram. Int., 2014, 40, 9095–9100 CrossRef CAS PubMed.
- J. X. Liu, D. F. Zhang, X. P. Pu, D. Y. Dong, P. Q. Cai and H. J. Seo, Mater. Lett., 2014, 130, 94–96 CrossRef CAS PubMed.
- J. Di, J. X. Xia, S. Yin, H. Xu, M. Q. He, H. M. Li, L. Xu and Y. P. Jiang, RSC Adv., 2013, 3, 19624–19631 RSC.
- Y. G. Xu, H. Xu, J. Yan, H. M. Li, L. Y. Huang, Q. Zhang, C. J. Huang and H. L. Wan, Phys. Chem. Chem. Phys., 2013, 15, 5821–5830 RSC.
- Y. G. Xu, M. Xie, T. Zhou, S. Yin, H. Xu, H. Y. Ji, H. M. Li and Q. Zhang, New J. Chem., 2015, 39, 5540–5547 RSC.
- P. Wang, B. B. Huang, Q. Q. Zhang, X. Y. Zhang, X. Y. Qin, Y. Dai, J. Zhan, J. X. Yu, H. X. Liu and Z. Z. Lou, Chem.–Eur. J., 2010, 16, 10042–10047 CrossRef CAS PubMed.
- Y. G. Xu, H. Xu, J. Yan, H. M. Li, L. Y. Huang, J. X. Xia, S. Yin and H. M. Shu, Colloids Surf., A, 2013, 436, 474–483 CrossRef CAS PubMed.
- S. L. Lin, L. Liu, J. S. Hu, Y. H. Liang and W. Q. Cui, Appl. Surf. Sci., 2015, 324, 20–29 CrossRef CAS PubMed.
- X. N. Liu, Q. F. Lu, C. F. Zhua and S. W. Liu, RSC Adv., 2015, 5, 4077–4082 RSC.
- S. Q. Huang, Y. G. Xu, M. Xie, H. Xu, M. Q. He, J. X. Xia, L. Y. Huang and H. M. Li, Colloids Surf., A, 2015, 478, 71–80 CrossRef CAS PubMed.
- S. K. Maji, N. Mukherjee, A. Mondal and B. Adhikary, Polyhedron, 2012, 33, 145–149 CrossRef CAS PubMed.
- J. Di, J. X. Xia, S. Yin, H. Xu, L. Xu, Y. G. Xu, M. Q. He and H. M. Li, J. Mater. Chem. A, 2014, 2, 5340–5351 CAS.
- Y. Sang, L. Kuai, C. Y. Chen, Z. Fang and B. Y. Geng, ACS Appl. Mater. Interfaces, 2014, 6, 5061–5068 CAS.
- Y. Zhang, J. Gu, M. Murugananthan and Y. R. Zhang, J. Alloys Compd., 2015, 630, 110–116 CrossRef CAS PubMed.
- H. Xu, J. Yan, Y. G. Xu, Y. H. Song, H. M. Li, J. X. Xia, C. J. Huang and H. L. Wan, Appl. Catal., B, 2013, 129, 182–193 CrossRef CAS PubMed.
- Y. Hou, F. Zuo, A. Dagg and P. Y. Feng, Nano Lett., 2012, 12, 6464–6473 CrossRef CAS PubMed.
- Y. G. Xu, M. Xie, S. Q. Huang, H. Xu, H. Y. Ji, J. X. Xia, Y. P. Li and H. M. Li, RSC Adv., 2015, 5, 26281–26290 RSC.
- Q. Xiang, J. Yu and M. Jaroniec, J. Phys. Chem. C, 2011, 115, 7355–7363 CAS.
- J. Jiang, X. Zhang, P. B. Sun and L. Z. Zhang, J. Phys. Chem. C, 2011, 115, 20555–20564 CAS.
- L. Z. Dong, Y. M. He, T. T. Li, J. Cai, W. D. Hu, S. S. Wang, H. J. Lin, M. F. Luo, X. D. Yi, L. H. Zhao, W. Z. Weng and H. L. Wan, Appl. Catal., A, 2014, 472, 143–151 CrossRef CAS PubMed.
- Y. P. Bi, S. X. Ouyang, J. Y. Cao and J. H. Ye, Phys. Chem. Chem. Phys., 2011, 13, 10071–10075 RSC.
- Y. J. Wang, Z. X. Wang, S. Muhammad and J. He, CrystEngComm, 2012, 14, 5065–5070 RSC.
- X. L. Xiao, L. Ge, C. C. Han, Y. J. Li, Z. Zhao, Y. J. Xin, S. Fang, L. Wu and P. Qiu, Appl. Catal., B, 2015, 163, 564–572 CrossRef CAS PubMed.
- P. H. Li, B. L. Li, Z. M. An, L. P. Mo, Z. S. Cui and Z. H. Zhang, Adv. Synth. Catal., 2013, 355, 2952–2959 CrossRef CAS PubMed.
- X. Xiao, R. Hao, M. Liang, X. X. Zuo, J. Nan, L. S. Li and W. D. Zhang, J. Hazard. Mater., 2012, 233, 122–130 CrossRef PubMed.
- X. J. Bai, L. Wang, Y. J. Wang, W. Q. Yao and Y. F. Zhu, Appl. Catal., B, 2014, 152, 262–270 CrossRef PubMed.
- J. G. Yu and B. Wang, Appl. Catal., B, 2010, 94, 295–302 CrossRef CAS PubMed.
- G. D. Yang, Z. Jiang, H. H. Shi, T. C. Xiao and Z. F. Yan, J. Mater. Chem., 2010, 20, 5301–5309 RSC.
- S. Linic, P. Christopher and D. B. Ingram, Nat. Mater., 2011, 10, 911–921 CrossRef CAS PubMed.
- H. F. Cheng, B. B. Huang, P. Wang, Z. Y. Wang, Z. Z. Lou, J. P. Wang, X. Y. Qin, X. Y. Zhang and Y. Dai, Chem. Commun., 2011, 47, 7054–7056 RSC.
- X. F. Zhou, C. Hu, X. X. Hu, T. W. Peng and J. H. Qu, J. Phys. Chem. C, 2010, 114, 2746–2750 CAS.
- J. Di, J. X. Xia, Y. P. Ge, H. P. Li, H. Y. Ji, H. Xu, Q. Zhang, H. M. Li and M. N. Li, Appl. Catal., B, 2015, 168, 51–61 CrossRef PubMed.
- C. C. Shen, Q. Zhu, Z. W. Zhao, T. Wen, X. K. Wang and A. W. Xu, J. Mater. Chem. A, 2015, 3, 14661–14668 CAS.
- H. G. Yu, R. Liu, X. F. Wang, P. Wang and J. G. Yu, Appl. Catal., B, 2012, 111, 326–333 CrossRef PubMed.
- Y. H. Liang, S. L. Lin, L. Liu, J. S. Hu and W. Q. Cui, Appl. Catal., B, 2015, 164, 192–203 CrossRef CAS PubMed.
- (a) M. S. A. S. Shah, W. Kim, J. Park, D. K. Rhee, I. H. Jang, N. G. Park, J. Y. Lee and P. J. Yoo, ACS Appl. Mater. Interfaces, 2014, 6, 20819–20827 CrossRef PubMed; (b) J. Tian, R. Liu, G. H. Wang, Y. Xu, X. Wang and H. Yu, Appl. Surf. Sci., 2014, 319, 324–331 CrossRef CAS PubMed; (c) C. Dong, K. L. Wu, X. W. Wei, J. Wang, L. Liu and B. B. Jiang, Appl. Catal., A, 2014, 488, 11–18 CrossRef CAS PubMed; (d) H. Xu, H. M. Li, J. X. Xia, S. Yin, Z. J. Luo, L. Liu and L. Xu, ACS Appl. Mater. Interfaces, 2011, 3, 22–29 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |