Acid/base-controlled chemodivergent synthesis of two differently functionalized tetrahydroimidazo[1,2-a]pyridines†
Wei-Si Guo,
Xing Xin,
Ke-Long Zhao,
Li-Rong Wen* and
Ming Li*
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science and Technology, Qingdao 266042, P. R. China. E-mail: wenlirong@qust.edu.cn; liming928@qust.edu.cn
First published on 12th August 2015
Abstract
A new approach for the facile chemodivergent synthesis of tetrahydroimidazo[1,2-a]pyridines from β-ketothioamides, aldehydes and heterocyclic ketene aminals is described. In the presence of a base, an unprecedented ring-opening, tautomerization, recyclization cascade is observed and H2S eliminated tetrahydroimidazo[1,2-a]pyridines were formed in good yields. With catalytic amounts of acid, another type of different substituted tetrahydroimidazo[1,2-a]pyridines were obtained through dehydration.
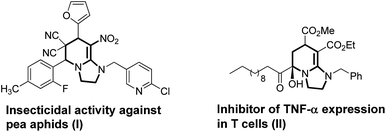
Imidazo[1,2-a]pyridine is an important heterocyclic skeleton which can be found in a broad spectrum of pharmaceuticals,1 such as insecticide against pea aphids (I),2 and the inhibitors of TNF-α expression in T cells (II) (Fig. 1).3 Therefore, a variety of methodologies have been developed for the construction of imidazo[1,2-a]pyridines.4 Recently, diverse metal-catalyzed methods for the construction of this privileged structure have been reported.5 Multicomponent reactions (MCRs) are an important strategy in the synthesis of heterocyclic scaffolds.6 The diversity and easy accessibility to a large number of compounds, make MCRs a very important tool in modern organic synthesis.7 However, there is only limited number of reports on imidazo[1,2-a]pyridines synthesis through multicomponent reactions under metal-free conditions.8
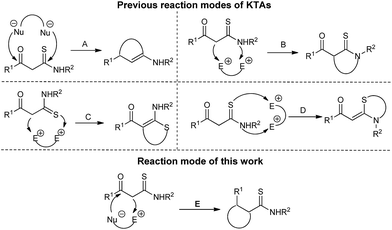
β-Ketothioamides (KTAs)9 and heterocyclic ketene aminals (HKAs)10 are versatile building blocks for the rapid construction of various heterocyclic compounds.11 The previous reports of KTAs participated reactions mainly involved four types (Fig. 2, modes A–D). In continuation of our interests in the utilization of HKAs and KTAs,12 and based on our recent works in exploring metal-free MCRs,13 herein, we report a metal-free acid/base controlled chemodivergent synthesis of tetrahydroimidazo[1,2-a]pyridines by another reaction mode of KTAs (Fig. 2, mode E).
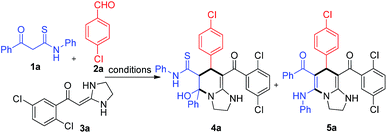
Initially, a mixture of KTA 1a, aldehyde 2a, and HKA 3a was stirred in refluxing toluene in the presence of Na2CO3 for 8 h, a yellow powder 5a was obtained in 28% yield after column chromatography (Table 1, entry 1). Then a series of other bases, including Et3N, DABCO, DBU, CH3ONa and K2CO3, were screened. Disappointedly, all these bases gave lower yields than Na2CO3 (entries 2–7). Subsequently, the model reaction was performed in various solvents. When the solvent was changed to methanol, another compound 4a was obtained besides 5a, albeit in low yield (entry 8). Interestingly, the selectivity of the reaction was changed from 5a to 4a without addition of any base (entry 9). Through carefully reaction conditions screening (entries 10–15), 4a was obtained in high yield in refluxing methanol (entry 10). Furthermore, we found that 4a could be transformed to 5a in high yield using Na2CO3 as a base in DMF. Fortunately, the structure of 4a was determined by single crystal X-ray analysis (see ESI†). Thus we reasoned that an interesting ring-opening, tautomerization, recyclization cascade could be involved in the transformation.
| Entry | Base | Solvent | Temp (°C) | Time (h) | Yieldb (%) | |
|---|---|---|---|---|---|---|
| 4a | 5a | |||||
| a The mixture of 1a (0.5 mmol), 2a (0.5 mmol), and 3a (0.5 mmol) was stirred in solvent at indicated temperature in the presence of air.b Isolated yield. | ||||||
| 1 | Na2CO3 | Toluene | 110 | 8 | Trace | 28 |
| 2 | Et3N | Toluene | 110 | 8 | Trace | 20 |
| 3 | DABCO | Toluene | 110 | 8 | Trace | 23 |
| 4 | Pyridine | Toluene | 110 | 8 | Trace | 18 |
| 5 | DBU | Toluene | 110 | 8 | Trace | 16 |
| 6 | CH3ONa | Toluene | 110 | 8 | Trace | 13 |
| 7 | K2CO3 | Toluene | 110 | 8 | Trace | 21 |
| 8 | Na2CO3 | MeOH | 65 | 8 | 23 | 15 |
| 9 | MeOH | 65 | 8 | 83 | Trace | |
| 10 | Toluene | 110 | 8 | 30 | Trace | |
| 11 | MeCN | 80 | 8 | 70 | Trace | |
| 12 | EtOH | 80 | 8 | 80 | Trace | |
| 13 | Dioxane | 100 | 8 | 50 | Trace | |
| 14 | MeOH | r.t. | 20 | Trace | Trace | |
| 15 | MeOH | 50 | 20 | 20 | Trace | |
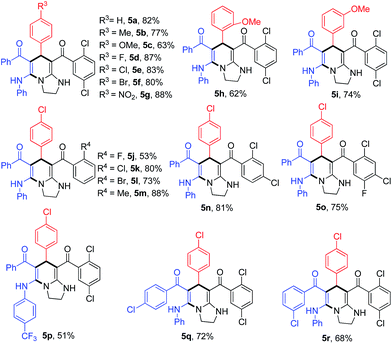
Under the optimal conditions, the scope of the reaction was examined using a broad range of substituted arylaldehydes (Table 2). Products 5a–i were obtained in moderate to good yields regardless of electron-withdrawing or electron-donating groups on the benzaldehydes. Next, substituted aryl–HKAs were employed to provide the desired products 5j–o in moderate to good yields. Interestingly, tetrahydroimidazo[1,2-a]pyridines 5 were just obtained from ortho substituted aryl–HKAs. No desired products were formed when non-, meta- or para-substituted aryl–HKAs were used as reactants. The reason for this phenomenon is still unclear. The reaction also proceeded smoothly with three other substituted KTAs to afford the tetrahydroimidazo[1,2-a]pyridines 5p–r. The structures of 5 were confirmed by X-ray crystallographic analysis of 5c (see ESI†).
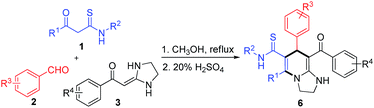
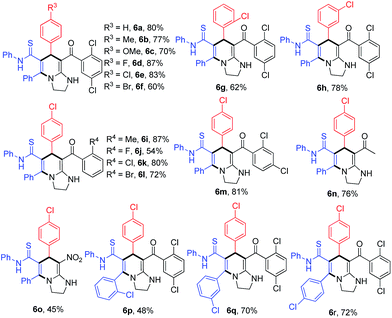
Under the conditions of catalytic sulfuric acid, another different substituted type of tetrahydroimidazo[1,2-a]pyridine 6a could be formed in high yield from intermediate 4a. The substrate scope of this reaction was also evaluated (Table 3). First, a variety of arylaldehydes 2 were reacted with HKA 1 and KTA 3, and products 6a–h were obtained in good yields. Subsequently, tetrahydroimidazo[1,2-a]pyridines 6i–m were formed in moderate to good yields with substituted aryl–HKAs. In addition, products 6n and 6o were also obtained when methyl–HKA and nitro–HKA were used as starting materials. Next, three substituted KTAs were employed with products 6q and 6r formed in good yields, whereas 6p was obtained in relatively low yield. The structures of 6 were confirmed by X-ray crystallographic analysis of 6h (see ESI†). Notably, the 1H NMR spectra of some compounds 6 (such as 6a–g) are messy due to rotamers when an ortho-chlorine atom was performed on the aromatic ring of HKAs 3. The reason for this phenomenon was explained in detail in our previous report.12a
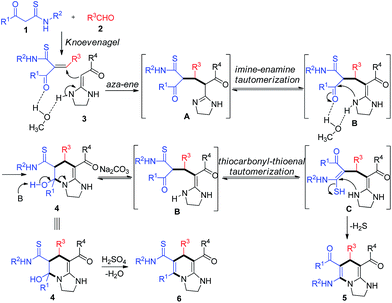
Based on the structure of the crucial intermediate 4, a plausible mechanism for the reactions is outlined in Scheme 1. Initially, the Knoevenagel adduct, which is readily formed in situ from aldehyde 2 and β-ketothioamide 3, reacts with HKA 1 via aza-ene process to form intermediate A. After imine–enamine tautomerization and intramolecular cyclization, the intermediate 4 is obtained. The formation of 4 is due to the α-carbon and the carbonyl group of KTAs participated in the reaction under neutral conditions. Notably, in this process, CH3OH would play an important role in increasing the electrophilicity of the carbonyl group of KTAs by hydrogen bond-driven effect,14 which is preferentially attacked by nucleophilic reagent giving the ring-closing product 4. Subsequently, in the presence of sodium carbonate, the semi-aminal group would collapse to regenerate the ring-open intermediate B. Then the thiocarbonyl group would tautomerize to form the more stable conjugated thioenol intermediate C under basic conditions. Finally, the tetrahydroimidazo[1,2-a]pyridine 5 is afforded irreversibly through intramolecular cyclization with elimination of H2S. Otherwise, in the presence of acid, the tetrahydroimidazo[1,2-a]pyridine 6 is obtained directly through dehydration.
In conclusion, a chemodivergent metal-free multicomponent synthesis of two differently functionalized tetrahydroimidazo[1,2-a]pyridine derivatives has been developed by adjusting reaction conditions. The ring-opening, tautomerization, recyclization cascade was also first observed in the study of KTAs reactions. The approach features high chemoselectivity, broad substrate scope, and mild reaction conditions, which provides a convenient method for construction of tetrahydroimidazo[1,2-a]pyridine derivatives from readily available starting materials.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21372137 and 21072110) and the Natural Science Foundation of Shandong Province (ZR2014BM006).Notes and references
- C. Enguehard-Gueiffier and A. Gueiffier, Mini-Rev. Med. Chem., 2007, 7, 888 CrossRef CAS.
- W. W. Zhang, Y. B. Chen, W. D. Chen, Z. W. Liu and Z. Li, J. Agric. Food Chem., 2010, 58, 6296 CrossRef CAS PubMed.
- J. Rether, G. Erkel, T. Anke, J. Bajtnerb and O. Sterner, Bioorg. Med. Chem., 2008, 16, 1236 CrossRef CAS PubMed.
- (a) E. F. Dimauro and J. M. Kennedy, J. Org. Chem., 2007, 72, 1013 CrossRef CAS PubMed; (b) S. Husinec, R. Markovic, M. Petkovic, V. Nasufovic and V. Savic, Org. Lett., 2011, 13, 2286 CrossRef CAS PubMed; (c) L. Ma, X. Wang, W. Yu and B. Han, Chem. Commun., 2011, 11333 RSC; (d) N. Shao, G. X. Pang, C. X. Yan, G. F. Shi and Y. Cheng, J. Org. Chem., 2011, 76, 7458 CrossRef CAS PubMed; (e) D. Sucunza, A. Samadi, M. Chioua, D. B. Silva, C. Yunta, L. Infantes, M. C. Carreiras, E. Soriano and J. Marco-Contelles, Chem. Commun., 2011, 5043 RSC; (f) D. K. Nair, S. M. Mobin and I. N. Namboothiri, Org. Lett., 2012, 14, 4580 CrossRef CAS PubMed; (g) C. He, J. Hao, H. Xu, Y. Mo, H. Liu, J. Han and A. Lei, Chem. Commun., 2012, 11073 RSC; (h) L. Guetzoyan, N. Nikbin, I. R. Baxendale and S. V. Ley, Chem. Sci., 2013, 4, 764 RSC.
- (a) N. Chernyak and V. Gevorgyan, Angew. Chem., Int. Ed., 2010, 49, 2743 CrossRef CAS PubMed; (b) R. L. Yan, H. Yan, C. Ma, Z. Y. Ren, X. A. Gao, G. S. Huang and Y. M. Liang, J. Org. Chem., 2012, 77, 2024 CrossRef CAS PubMed; (c) J. Zeng, Y. J. Tan, M. L. Leow and X. W. Liu, Org. Lett., 2012, 14, 4386 CrossRef CAS PubMed; (d) Z. J. Cai, S. Y. Wang and S. J. Ji, Adv. Synth. Catal., 2013, 355, 2686 CrossRef CAS PubMed; (e) S. Santra, A. K. Bagdi, A. Majee and A. Hajra, Adv. Synth. Catal., 2013, 355, 1065 CrossRef CAS PubMed; (f) D. C. Mohan, S. N. Rao and S. Adimurthy, J. Org. Chem., 2013, 78, 1266 CrossRef PubMed; (g) Y. Wang, B. Frett and H. Y. Li, Org. Lett., 2014, 16, 3016 CrossRef CAS PubMed; (h) E. P. A. Talbot, M. Richardson, J. M. McKenna and F. D. Toste, Adv. Synth. Catal., 2014, 356, 687 CrossRef CAS PubMed; (i) K. Monir, A. K. Bagdi, S. Mishra, A. Majee and A. Hajra, Adv. Synth. Catal., 2014, 356, 1105 CrossRef CAS PubMed; (j) H. Yan, Y. Wang, C. Pan, H. Zhang, S. Yang, X. Ren, J. Li and G. Huang, Eur. J. Org. Chem., 2014, 2754 CrossRef CAS PubMed.
- For selected reviews, see: (a) J. P. Zhu and H. Bienaymé, Multicomponent Reactions, Wiley-VCH, Weinheim, 2005 Search PubMed; (b) A. Domling, Chem. Rev., 2006, 106, 17 CrossRef PubMed; (c) A. Domling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083 CrossRef CAS PubMed; (d) C. de Graaff, E. Ruijter and R. V. A. Orru, Chem. Soc. Rev., 2012, 41, 3969 RSC; (e) V. Estevez, M. Villacampa and J. C. Menendez, Chem. Soc. Rev., 2014, 43, 4633 RSC.
- (a) G. Qiu, Y. He and J. Wu, Chem. Commun., 2012, 48, 3836 RSC; (b) X. Feng, Q. Wang, W. Lin, G. L. Dou, Z. B. Huang and D. Q. Shi, Org. Lett., 2013, 15, 2452 Search PubMed; (c) J. S. Alford and H. M. L. Davies, J. Am. Chem. Soc., 2014, 136, 10266 CrossRef CAS PubMed; (d) P. Mampuys, Y. Zhu, T. Vlaar, E. Ruijter, R. V. A. Orru and B. U. W. Maes, Angew. Chem., Int. Ed., 2014, 53, 12849 CrossRef CAS PubMed; (e) G. H. Ma, B. Jiang, X. J. Tu, Y. Ning, S. J. Tu and G. Li, Org. Lett., 2014, 16, 4504 CrossRef CAS PubMed; (f) Y. Han, Y. J. Sheng and C. G. Yan, Org. Lett., 2014, 16, 2654 CrossRef CAS PubMed; (g) X. Feng, J. J. Wang, Z. Xun, J. J. Zhang, Z. B. Huang and D. Q. Shi, Chem. Commun., 2015, 51, 1528 RSC.
- H. Cao, X. Liu, L. Zhao, J. Cen, J. Lin, Q. Zhu and M. Fu, Org. Lett., 2014, 16, 146 CrossRef CAS PubMed.
- For a selected review, see: W. S. Guo, L. R. Wen and M. Li, Org. Biomol. Chem., 2015, 13, 1942 CAS.
- For a selected review, see: K. M. Wang, S. J. Yan and J. Lin, Eur. J. Org. Chem., 2014, 1129 CrossRef CAS PubMed.
- For selected examples, see: (a) S. Yan, Y. Chen, L. Liu, N. He and J. Lin, Green Chem., 2010, 12, 2043 RSC; (b) F. Yu, S. Yan, L. Hu, Y. Wang and J. Lin, Org. Lett., 2011, 13, 4782 CrossRef CAS PubMed; (c) L. R. Wen, Z. R. Li, M. Li and H. Cao, Green Chem., 2012, 14, 707 RSC; (d) F. Yu, R. Huang, H. Ni, J. Fan, S. Yan and J. Lin, Green Chem., 2013, 15, 453 RSC.
- (a) M. Li, P. Shao, S. W. Wang, W. Kong and L. R. Wen, J. Org. Chem., 2012, 77, 8956 CrossRef CAS PubMed; (b) L. R. Wen, Q. C. Sun, H. L. Zhang and M. Li, Org. Biomol. Chem., 2013, 11, 781 RSC; (c) L. R. Wen, T. He, M. C. Lan and M. Li, J. Org. Chem., 2013, 78, 10617 CrossRef CAS PubMed; (d) L. R. Wen, L. B. Men, T. He, G. J. Ji and M. Li, Chem.–Eur. J., 2014, 20, 5028 CrossRef CAS PubMed.
- (a) M. Li, H. Cao, Y. Wang, X. L. Lv and L. R. Wen, Org. Lett., 2012, 14, 3470 CrossRef CAS PubMed; (b) M. Li, X. L. Lv, L. R. Wen and Z. Q. Hu, Org. Lett., 2013, 15, 1262 CrossRef CAS PubMed.
- R. Chebolu, D. N. Kommi, D. Kumar, N. Bollineni and A. K. Chakraborti, J. Org. Chem., 2012, 77, 10158 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, full spectroscopic data for all new compounds, and crystal data for 4a, 5c and 6h (CIF). CCDC 858868, 878006 and 1003512. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra13395e |
| This journal is © The Royal Society of Chemistry 2015 |