Low band-gap polymers based on easily synthesized thioester-substituted thieno[3,4-b]thiophene for polymer solar cells
Dangqiang Zhuab,
Liang Suna,
Xichang Baoa,
Shuguang Wena,
Liangliang Hana,
Chuantao Guab,
Jing Guoa and
Renqiang Yang*ac
aCAS Key Laboratory of Bio-based Materials, Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, Qingdao 266101, China. E-mail: yangrq@qibebt.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
cState Key Laboratory of Luminescent Materials and Devices, South China University of Technology, Guangzhou 510641, China
First published on 14th July 2015
Abstract
A new acceptor S-alkyl thieno[3,4-b]thiophene-2-carbothioate-based acceptor (TTS) was firstly developed via easy of synthesis and applied in the construction of donor–acceptor (D–A) type conjugated polymers, by replacing the alkyl chain of ketone-substituted thieno[3,4-b]thiophene with an alkylthio side chain. Then, two new TTS-based polymers PBDTT-TTSO and PBDTT-TTSE were synthesized by Stille coupling reaction. The TTS acceptor moieties made the polymers exhibit a lower band gap (∼1.5 eV) and appropriate HOMO and LUMO energy levels relative to the fullerene acceptors, which could make the polymers perform well in photovoltaic devices. Solar cells were fabricated with the structure ITO/PEDOT:PSS/polymer:PC71BM/Ca/Al. The polymer PBDTT-TTSO device exhibits a power conversion efficiency (PCE) of 4.7% with an open circuit voltage (VOC) of 0.68 V, a short circuit current density (JSC) of 12.7 mA cm−2, and a fill factor (FF) of 54.6% while the PBDTT-TTSE device yields a higher PCE of 5.8% with a VOC of 0.70 V, a JSC of 14.6 mA cm−2, and a FF of 56.7%. The results indicate that thioester-substituted thieno[3,4-b]thiophene (TTS) is a promising building block for further design of high performance photovoltaic polymers.
Introduction
Polymer solar cells (PSCs) have attracted much attention because of their potential utility in the production of flexible, light-weight, and low-cost large-area devices through roll-to-roll printing via solution processing.1–9 Although the maximum power conversion efficiency (PCE) of the PSCs has been over 10% for single junction solar cells,10 it still can't meet the demand of commercialization. Generally, in a typical bulk heterojunction (BHJ) PSC, the active layer consists of an interpenetrating network formed by an electron-donor material blended with an electron-acceptor fullerene derivative (typically [6,6]-phenyl-C61-butyric acid methyl ester (PC61BM) or [6,6]-phenyl-C71-butyric acid methyl ester (PC71BM)).11,12 Currently, the most critical challenge in developing ideal electron-donor materials is to design and synthesize conjugated polymers that simultaneously possess broad and strong absorption band in visible and near-infrared region, high hole mobility, suitable energy levels, good solubility for solution processing, optimal morphology and nanoscale phase separation of the interpenetrating network of the donor/acceptor blend active layer.13Conjugated polymers based on thieno[3,4-b]thiophene (TT) have been studied extensively as low optical band-gap polymers.14–16 However, compared with other donor–acceptor (D–A) polymers, the polymers based TT as A building block usually exhibit a lower open circuit voltage (VOC), which is the limiting factor for the device's PCE. Various electron-withdrawing groups such as ester (COOR),17–20 ketone (COR),21–23 sulfonyl (SO2R),24–26 fluorine (F),27,28 trifluoromethyl group (CF3),29 N-alkyl-2,7-dithia-5-azacyclopenta[a]pentalene-4,6-dione (DTPD)30 have been introduced on the 2 or 3 position of TT to tune the highest occupied molecular orbital (HOMO) level to improve VOC.
Moreover, there are only several literatures investigated alkylthio-substituent conjugated polymers so far.31–37 In contrast with alkyl chains, alkylthio substituents are of particular interest for several reasons. First, the van der Waals radius of sulfur (∼0.18 nm) is less than that of –CH2 (∼0.20 nm), which will reduce the steric strain slightly.31 Secondly, the sulfur atom is known to be a weak electron donor due to the poor overlap of its orbital with the π-system on the polymer backbone, which will decrease the HOMO and lowest unoccupied molecular orbital (LUMO) levels of the polymer.33,37 For example, according to the previous report,33 poly[(3-hexyl-thio)thiophene] (P3HST) showed a lower optical band gap of 1.82 eV compared with poly(3-hexylthiophene) (P3HT, 1.85 eV), and the alkylthio side chains decreased the HOMO energy level of the polymer. However, most alkylthio side chains reported were substituted on the D unit of the D–A type conjugated polymers in the form of thioether. Moreover, a potential problem may exist that sulfur atom in thioether compound can be oxidized easily, which may result in an unpredictable effect to the polymer.
Based on the above consideration, we replaced the alkyl chain of ketone-substituted TT with alkylthio side chain to develop a new acceptor S-alkyl thieno[3,4-b]thiophene-2-carbothioate (TTS). To our knowledge, the thioester substituent on the acceptor unit has not been reported. Apart from broadening the optical absorption and decreasing the HOMO energy level as previously mentioned, the antioxidant capacity of sulfur atom of thioester can be enhanced. In addition, compared with the TT-based acceptors mentioned previously, it is easier to synthesize monomer TTS, and the homologous polymers obtained exhibit promising photovoltaic performance. In this work, we reported the synthesis and characterization of two new polymers PBDTT-TTSO and PBDTT-TTSE, which consisted of benzodithiophene (BDT) and TTS moieties substituted with octyl or 2-ethylhexyl groups, respectively. Both polymers utilize light absorption to exceed 800 nm. Meanwhile, the two polymers show favorably aligned HOMO and LUMO energy levels relative to that of conventional fullerene acceptors. The photovoltaic performance of PBDTT-TTSE devices display a VOC of 0.70 V, a short circuit current density (JSC) of 14.6 mA cm−2, a fill factor (FF) of 56.7%, and a PCE of 5.80%. This work demonstrates that the new thioester-substituted acceptor TT moiety is a promising building block for high performance PSCs.
Results and discussion
Monomers and polymers synthesis
The synthetic routes for monomers and polymers are shown in Scheme 1.38,39 Firstly, 3,4-dibromothiophene was mono-lithiated by n-BuLi and subsequently quenched with dimethylformamide (DMF) to produce compound 2 in 82% yield. Then a mixture of compound 2, ethyl thioglycolate and potassium carbonate was heated at 80 °C for 12 h with copper(II) oxide nanopowder as the catalyst to provide thieno[3,4-b]thiophene-2-carboxylate (3) in 54% yield. Compound 3 was brominated with N-bromosuccinimide (NBS) at room temperature to give compound 4 in 70% yield. After hydrolysis in the alkali medium, the corresponding TT carboxylic acid was obtained. Finally, the monomer 6 can be obtained by the condensation of the carboxylic acid and the corresponding thioalcohol (RSH).The polymers PBDTT-TTSO and PBDTT-TTSE were synthesized by Stille coupling reaction with tetrakis(triphenylphosphine)palladium(0) (Pd(PPh3)4) as the catalyst. The crude products were precipitated in methanol and collected by filtration. Soxhlet purification afforded the black polymers. Gel permeation chromatography (GPC) analysis using tetrahydrofuran as an eluent provided a number-average molecular weight (Mn) of 18.5 kDa with a polydispersity index (PDI) of 2.01 for PBDTT-TTSO and a Mn of 25.3 kDa with a PDI of 1.88 for PBDTT-TTSE, with respect to a polystyrene standard.
Thermal properties
The thermal properties of the polymers were examined by thermogravimetric analysis (TGA) and the TGA curves are shown in Fig. 1. In the TGA measurement, the decomposition temperatures (Td) at a 5% weight loss occurred under a nitrogen atmosphere are as high as 374 °C and 380 °C for PBDTT-TTSO and PBDTT-TTSE, respectively, which show good thermal stabilities. It is adequate for the fabrication of PSCs and other optoelectronic devices. | ||
| Fig. 1 TGA plots of PBDTT-TTSO and PBDTT-TTSE with a heating rate of 10 °C min−1 under the protection of nitrogen. | ||
Optical properties
The ultraviolet-visible (UV-vis) absorption spectra of the polymers PBDTT-TTSO and PBDTT-TTSE in dilute chloroform solution and as thin films are shown in Fig. 2. The optical properties of the polymers are listed in Table 1. Both polymers show very similar optical characteristics, covering a broad absorption range from 300 nm to 800 nm. The absorption peaks at short wavelength are originated from π–π* transition of the polymer main chains and side chains, while the absorption peaks at long wavelength could be attributed to the strong intramolecular charge transfer (ICT) between the electron-rich moieties and electron-deficient segments in the polymer backbones.40,41 The absorption peak of the polymer PBDTT-TTSO has a red shift of 19 nm as thin film compared to the absorption peak of 644 nm in dilute chloroform solution. Meanwhile, PBDTT-TTSE shows the absorption peak at 661 nm and an enhanced band at 712 nm in solid film, which indicates slightly increased π–π stacking. The absorption edges of PBDTT-TTSO and PBDTT-TTSE are at 816 and 827 nm corresponding to the optical band gaps of 1.52 and 1.50 eV, respectively. The optical band gaps are lower than those of ester or ketone-substituted polymer PBDTTT-E-T (1.58 eV) and PBDTTT-C-T (1.58 eV).22 The results also support the viewpoint that the alkylthio side chain can broaden the optical absorption mentioned previously.33,34,36 | ||
| Fig. 2 UV-vis absorption spectra of PBDTT-TTSO and PBDTT-TTSE in dilute chloroform solution (a) and in the film (b). | ||
Electrochemical properties
Electrochemical cyclic voltammetry (CV) was carried out to measure the molecular energy levels of the polymers.42 The redox potentials are estimated from the onsets of the oxidation and reduction. All voltammograms were calibrated using the ferrocene/ferrocenium (Fc/Fc+) redox couple as the standard, and their energy levels are assumed at −4.8 eV relative to vacuum. The potential of this internal standard under the same condition was 0.39 eV versus saturated calomel electrode (SCE). As shown in Fig. 3a, the onsets of oxidation and reduction of PBDTT-TTSO are observed at +0.92 and −0.70 V vs. SCE. The corresponding HOMO and LUMO energy levels are estimated to be −5.33 and −3.71 eV according to the empirical equation: EHOMO/LUMO = −(Eox/red + 4.41) eV. Similarly, the HOMO and LUMO energy levels of PBDTT-TTSE are determined to be −5.36 and −3.73 eV (Table 1). The HOMO energy levels of PBDTT-TTSO and PBDTT-TTSE calculated from CV curves are similar to that of DTPD-based polymers PEBDTPD (−5.37 eV).30 Although the branched side chain can slightly decrease the HOMO level compared to the linear side chain in accordance with that of ester or DTPD-substituted polymers reported,17,30 the energy levels of both polymers are in the ideal range for the high PCEs according to Brabec's estimation if taking the LUMO energy level of PC71BM as −4.1 eV (Fig. 3b).43 As well known, the VOC is mainly dependent on the difference between the HOMO energy level of the donor and the LUMO energy level of the fullerene acceptor, the relatively deep-lying HOMO levels of two polymers could give a high VOC in solar cells.Photovoltaic performance
In order to investigate the potential applications of both polymers in solar cells, the BHJ-PSCs were fabricated with device structure of ITO/PEDOT:PSS/polymer:PCBM/Ca/Al, where PEDOT:PSS and Ca layers were employed as buffer layers to facilitate hole and electron extractions. The devices were tested under the illumination of AM 1.5G, 100 mW cm−2. The current density–voltage curves of the polymer/PC71BM devices are shown in Fig. 4a and the optimized photovoltaic performance with 1,8-diiodooctane (DIO, 3%, v/v) are listed in Table 2. The device based on PBDTT-TTSO/PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, w/w) exhibits a VOC of 0.67 V, a JSC of 11.8 mA cm−2 and a FF of 54.7%, yielding a PCE of 4.33%. When replacing PC61BM with PC71BM, the JSC can increase to 12.7 mA cm−2 while the VOC and FF almost remain unchanged. In comparison, the VOC of PBDTT-TTSE device is ∼0.02 V higher than that of PBDTT-TTSO, which is well consistent with their HOMO energy levels (−5.33 eV and −5.36 eV, respectively) (Table 1). The optimized photovoltaic performance of PBDTT-TTSE/PC71BM demonstrates a VOC of 0.70 V, a JSC of 14.6 mA cm−2, a FF of 56.7%, and a PCE of 5.80%, which is almost the same as that of non-fluorine substituted TT-based polymers including ester-substituted polymer PBDTTT-E-T (6.21%),22 DTPD-based polymers PEBDTPD (5.30%),30 and sulfonyl-based polymers PBDTTT-S-T (5.93%).26
1.5, w/w) exhibits a VOC of 0.67 V, a JSC of 11.8 mA cm−2 and a FF of 54.7%, yielding a PCE of 4.33%. When replacing PC61BM with PC71BM, the JSC can increase to 12.7 mA cm−2 while the VOC and FF almost remain unchanged. In comparison, the VOC of PBDTT-TTSE device is ∼0.02 V higher than that of PBDTT-TTSO, which is well consistent with their HOMO energy levels (−5.33 eV and −5.36 eV, respectively) (Table 1). The optimized photovoltaic performance of PBDTT-TTSE/PC71BM demonstrates a VOC of 0.70 V, a JSC of 14.6 mA cm−2, a FF of 56.7%, and a PCE of 5.80%, which is almost the same as that of non-fluorine substituted TT-based polymers including ester-substituted polymer PBDTTT-E-T (6.21%),22 DTPD-based polymers PEBDTPD (5.30%),30 and sulfonyl-based polymers PBDTTT-S-T (5.93%).26
 | ||
Fig. 4 (a) The J–V curves and (b) EQE spectra of the PSCs based on polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1 PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) with 3% DIO (v/v). 1.5) with 3% DIO (v/v). | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM = 1
PCBM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 (w/w) with 3%DIO (v/v)
1.5 (w/w) with 3%DIO (v/v)
EQE spectra were measured under illumination of monochromatic light shown in Fig. 4b. The EQE curves of the devices show a good photo response in the 300–800 nm regions, which coincides with the corresponding absorption spectra. It is observed that the EQE value of PBDTT-TTSE is higher than that of PBDTT-TTSO in the range (430–800 nm). The highest value for PBDTT-TTSE can exceed 60% at 460 and 650 nm. The result agrees well with the higher JSC and PCE of PBDTT-TTSE than PBDTT-TTSO.
The hole mobilities of the polymers were investigated by the space charge limited current (SCLC) method. High mobility could guarantee effective charge carrier transport to the electrodes and reduce the photocurrent loss in photovoltaic devices. The hole only mobilities of the blend films were measured with a device structure of ITO/PEDOT:PSS/polymer:PC71BM (100 nm)/Au. The SCLC model is described by
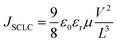 | (1) |
Morphological characterization
Atomic force microscopy (AFM) was employed to investigate the surface morphology of the blend films of polymer/PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, w/w) with the addictive of 3% DIO.44 The height and phase images are shown in Fig. 6 and the preparation conditions are the same as the device fabrication. AFM images of both blend films exhibit smooth surfaces while the root-mean-squared (RMS) roughness of the PBDTT-TTSO/PC71BM film (RMS = 2.64 nm) was slightly lower than that of the PBDTT-TTSE/PC71BM film (RMS = 3.13 nm). For the phase image, the dark-colored and light-colored areas correspond to PC71BM domains and polymers, respectively. As we know the exiton diffusion length is less than 20 nm and the perfect domain size should not exceed 30 nm.45 It can be observed from Fig. 6b and d that the phase images of the blend films exhibit nanoscale phase separation with suitable domain sizes, which could contribute to charge carrier transport and collection. Interesting, the polymer PBDTT-TTSE shows a more desirable nanoscale phase separation with the domain sizes of ∼20 nm compared to PBDTT-TTSO. The results are in accord with the photovoltaic performance of the two polymers.
1.5, w/w) with the addictive of 3% DIO.44 The height and phase images are shown in Fig. 6 and the preparation conditions are the same as the device fabrication. AFM images of both blend films exhibit smooth surfaces while the root-mean-squared (RMS) roughness of the PBDTT-TTSO/PC71BM film (RMS = 2.64 nm) was slightly lower than that of the PBDTT-TTSE/PC71BM film (RMS = 3.13 nm). For the phase image, the dark-colored and light-colored areas correspond to PC71BM domains and polymers, respectively. As we know the exiton diffusion length is less than 20 nm and the perfect domain size should not exceed 30 nm.45 It can be observed from Fig. 6b and d that the phase images of the blend films exhibit nanoscale phase separation with suitable domain sizes, which could contribute to charge carrier transport and collection. Interesting, the polymer PBDTT-TTSE shows a more desirable nanoscale phase separation with the domain sizes of ∼20 nm compared to PBDTT-TTSO. The results are in accord with the photovoltaic performance of the two polymers.
 | ||
Fig. 6 AFM height (a, c, 4 μm × 4 μm) and phase images (b, d, 1 μm × 1 μm) of polymer: PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) blend films: (a and b) PBDTT-TTSO, (c and d) PBDTT-TTSE, respectively. 1.5) blend films: (a and b) PBDTT-TTSO, (c and d) PBDTT-TTSE, respectively. | ||
Experimental section
Materials
All starting materials and reagents were purchased from commercial sources and used without further purification, unless otherwise mentioned. Toluene was distilled over sodium in the presence of benzophenone as indicator. CHCl3 and CH2Cl2 were distilled over calcium hydride.Instrumentation
1H and 13C NMR spectra were measured on a Bruker Advance III 600 spectrometer with tetramethylsilane (TMS, δ = 0 ppm) as an internal standard. GPC analyses were made using tetrahydrofuran (THF) as eluant and polystyrene standard as reference. UV-vis absorption spectra were performed on Lambda25 spectrophotometer. Cyclic voltammetry (CV) measurements were taken on a CHI660D electrochemical workstation. The CV experiments were carried out at room temperature with a conventional three-electrode system using a glassy carbon electrode as working electrode, Pt wire as the counter electrode, and saturated calomel electrode as the reference electrode. Tetrabutylammonium phosphorus hexafluoride (Bu4NPF6, 0.1 M) in acetonitrile solution was used as the supporting electrolyte, and the scan rate was 100 mV s−1. Ferrocene/ferrocenium (Fc/Fc+) was used as the internal standard. Thermal gravimetric analysis (TGA) measurements were performed on STA-409 at a heating rate of 10 °C min−1. Topographic images of the active layers were obtained through atomic force microscopy (AFM) in tapping mode under ambient conditions using an Agilent 5400 instrument.Photovoltaic device fabrication
The PSCs devices were fabricated with a configuration of ITO/PEDOT:PSS/polymers:PCBM/Ca/Al. A thin layer of PEDOT:PSS [30 nm, Baytron PVP AI 4083] was spin-cast on precleaned ITO-coated glass at 4000 rpm. After baking at 150 °C for 20 min, the substrates were transferred into glovebox, where the active layer (∼100 nm) of the blend of the polymer and PCBM was spin-coated on the PEDOT:PSS layer. Finally, a Ca (10 nm)/Al (100 nm) metal top electrode was thermal evaporated onto the active layer under about 2 × 10−4 Pa. The active area of the device was 0.1 cm2 defined by shadow mask. The current density–voltage (J–V) characteristics were measured with a Keithley 2420 source measurement unit under simulated 100 mW cm−2 (AM 1.5G) irradiation from a Newport solar simulator. Light intensity was calibrated with a standard silicon solar cell. The external quantum efficiencies (EQE) of solar cells were analyzed using a certified Newport incident photon conversion efficiency measurement system.Monomer synthesis
S-Octyl 4,6-dibromothieno[3,4-b]thiophene-2-carbothioate (TTSO), pale yellow solid, yield: 53%. 1H NMR (600 MHz, CDCl3), δ (ppm): 7.52 (s, 1H), 3.09 (q, J = 7.2 Hz, 2H), 1.70–1.65 (m, 2H), 1.44–1.39 (m, 2H), 1.36–1.15 (m, 8H), 0.88 (t, J = 7.2 Hz, 3H). 13C NMR (150 MHz, CDCl3), δ (ppm): 185.7, 148.7, 145.5, 139.8, 120.6, 103.2, 97.3, 32.8, 29.6, 29.4, 29.2, 29.1, 28.9, 22.6, 14.1.
S-(2-Ethylhexyl) 4,6-dibromothieno[3,4-b]thiophene-2-carbothioate (TTSE), oil, yield: 58%. 1H NMR (600 MHz, CDCl3), δ (ppm): 7.53 (s, 1H), 3.17–3.11 (m, 2H), 1.64–1.59 (m, 1H), 1.45–1.26 (m, 8H), 0.94–0.87 (m, 6H). 13C NMR (150 MHz, CDCl3), δ (ppm): 185.7, 148.7, 145.6, 139.6, 120.5, 103.0, 97.4, 39.3, 33.5, 32.6, 28.9, 25.9, 22.9, 14.1, 10.9.
General method of polymerization. Bis(trimethyltin) BDT monomer (0.20 mmol), compound 6 (0.20 mmol) were mixed in 4 mL toluene and 1 mL DMF. After being purged with argon for 15 min, Pd (PPh3)4 (11.5 mg) was added as the catalyst, and the mixture was then purged with argon for another 25 min. The reaction mixture was refluxed for 24 h. Then the reaction mixture was cooled to room temperature, and the polymer was precipitated by addition of 50 mL methanol, filtered through a Soxhlet thimble. The precipitate was then subjected to Soxhlet extraction with methanol, hexane and chloroform. The polymer was recovered as solid from the chloroform fraction by precipitation from methanol. The black solid was dried under vacuum to afford the titled polymer.
PBDTT-TTSO, black solid, yield: 87%, 1H NMR (600 MHz, CDCl3), δ (ppm): 8.06–6.69 (m, 7H), 3.41–2.66 (m, 6H), 2.01–1.22 (m, 30H), 1.15–0.76 (m, 15H).
PBDTT-TTSE, black solid, yield: 93%, 1H NMR (600 MHz, CDCl3), δ (ppm): 8.13–6.63 (m, 7H), 3.63–2.65 (m, 6H), 1.97–1.22 (m, 27H), 1.19–0.73 (m, 18H).
Conclusion
In summary, we replaced the alkyl chain of ketone-substituted thieno[3,4-b]thiophene with alkylthio side chain to develop a new acceptor thioester-based acceptor (TTS), which was synthesized easily. Then, two new polymers PBDTT-TTSO and PBDTT-TTSE were designed and synthesized by Stille coupling reaction. The TTS acceptor moieties made the two polymers exhibit a lower band gap (∼1.5 eV) and desirable HOMO and LUMO energy levels relative to the fullerene acceptors for PSCs. The PBDTT-TTSE-based photovoltaic device demonstrates a VOC of 0.70 V, a JSC of 14.6 mA cm−2, a FF of 56.7%, and a high PCE of 5.80%. This study indicates that thioester substituted thieno[3,4-b]thiophene may be a highly useful building block for further design of high performance photovoltaic polymers.Acknowledgements
The authors are deeply grateful to the National Natural Science Foundation of China (21202181, 21274161, 51173199, 61107090), the Ministry of Science and Technology of China (2014CB643501, 2010DFA52310), and Qingdao Municipal Science and Technology Program (11-2-4-22-hz) for financial support.Notes and references
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789–1791 CAS.
- P. M. Beaujuge and J. M. Frechet, J. Am. Chem. Soc., 2011, 133, 20009–20029 CrossRef CAS PubMed.
- H. J. Son, B. Carsten, I. H. Jung and L. Yu, Energy Environ. Sci., 2012, 5, 8158–8170 CAS.
- P.-L. T. Boudreault, A. Najari and M. Leclerc, Chem. Mater., 2011, 23, 456–469 CrossRef CAS.
- D. Zhu and R. Yang, J. Nano Energy Power Res., 2013, 2, 73–91 CrossRef PubMed.
- P.-L. T. Boudreault, S. Beaupré and M. Leclerc, Polym. Chem., 2010, 1, 127–136 RSC.
- J.-T. Chen and C.-S. Hsu, Polym. Chem., 2011, 2, 2707–2722 RSC.
- X. Zhan and D. Zhu, Polym. Chem., 2010, 1, 409–419 RSC.
- H.-L. Yip and A. K. Y. Jen, Energy Environ. Sci., 2012, 5, 5994–6011 CAS.
- Y. Liu, J. Zhao, Z. Li, C. Mu, W. Ma, H. Hu, K. Jiang, H. Lin, H. Ade and H. Yan, Nat. Commun., 2014, 5, 5293 CrossRef CAS PubMed.
- C. Winder and N. S. Sariciftci, J. Mater. Chem., 2004, 14, 1077–1086 RSC.
- C.-Z. Li, H.-L. Yip and A. K. Y. Jen, J. Mater. Chem., 2012, 22, 4161–4177 RSC.
- Y. Li, Acc. Chem. Res., 2012, 45, 723–733 CrossRef CAS PubMed.
- Y. Liang and L. Yu, Acc. Chem. Res., 2010, 43, 1227–1236 CrossRef CAS PubMed.
- L. Huo and J. Hou, Polym. Chem., 2011, 2, 2453–2461 RSC.
- J. M. Szarko, B. S. Rolczynski, S. J. Lou, T. Xu, J. Strzalka, T. J. Marks, L. Yu and L. X. Chen, Adv. Funct. Mater., 2014, 24, 10–26 CrossRef CAS PubMed.
- Y. Liang, D. Feng, Y. Wu, S.-T. Tsai, L. Gang, C. Ray and L. Yu, J. Am. Chem. Soc., 2009, 131, 7792–7799 CrossRef CAS PubMed.
- Y. Liang, Y. Wu, D. Feng, S.-T. Tsai, H.-J. Son, L. Gang and L. Yu, J. Am. Chem. Soc., 2009, 131, 56–57 CrossRef CAS PubMed.
- Y. Liang, Z. Xu, J. Xia, S. T. Tsai, Y. Wu, G. Li, C. Ray and L. Yu, Adv. Mater., 2010, 22, E135–E138 CrossRef CAS PubMed.
- Q. Xu, F. Wang, D. Qian, Z. Tan, L. Li, S. Li, X. Tu, G. Sun, X. Hou, J. Hou and Y. Li, ACS Appl. Mater. Interfaces, 2013, 5, 6591–6597 CAS.
- J. Hou, H.-Y. Chen, S. Zhang, R. I. Chen, Y. Yang, Y. Wu and G. Li, J. Am. Chem. Soc., 2009, 131, 15586–15587 CrossRef CAS PubMed.
- L. Huo, S. Zhang, X. Guo, F. Xu, Y. Li and J. Hou, Angew. Chem., Int. Ed., 2011, 50, 9697–9702 CrossRef CAS PubMed.
- Z.-G. Zhang, H. Li, Z. Qi, Z. Jin, G. Liu, J. Hou, Y. Li and J. Wang, Appl. Phys. Lett., 2013, 102, 143902 CrossRef PubMed.
- Y. Huang, L. Huo, S. Zhang, X. Guo, C. C. Han, Y. Li and J. Hou, Chem. Commun., 2011, 47, 8904–8906 RSC.
- Y. Huang, X. Guo, F. Liu, L. Huo, Y. Chen, T. P. Russell, C. C. Han, Y. Li and J. Hou, Adv. Mater., 2012, 24, 3383–3389 CrossRef CAS PubMed.
- Y. Wu, Z. Li, W. Ma, Y. Huang, L. Huo, X. Guo, M. Zhang, H. Ade and J. Hou, Adv. Mater., 2013, 25, 3449–3455 CrossRef CAS PubMed.
- M. Zhang, X. Guo, S. Zhang and J. Hou, Adv. Mater., 2014, 26, 1118–1123 CrossRef CAS PubMed.
- M. Zhang, X. Guo, W. Ma, S. Zhang, L. Huo, H. Ade and J. Hou, Adv. Mater., 2014, 26, 2089–2095 CrossRef CAS PubMed.
- P. Deng, Z. Wu, K. Cao, Q. Zhang, B. Sun and S. R. Marder, Polym. Chem., 2013, 4, 5275–5282 RSC.
- S.-O. Kim, Y.-S. Kim, H.-J. Yun, I. Kang, Y. Yoon, N. Shin, H. J. Son, H. Kim, M. J. Ko, B. Kim, K. Kim, Y.-H. Kim and S.-K. Kwon, Macromolecules, 2013, 46, 3861–3869 CrossRef CAS.
- J. A. Schneider, A. Dadvand, W. Wen and D. F. Perepichka, Macromolecules, 2013, 46, 9231–9239 CrossRef CAS.
- C. Cui, W.-Y. Wong and Y. Li, Energy Environ. Sci., 2014, 7, 2276–2284 CAS.
- L. Huo, Y. Zhou and Y. Li, Macromol. Rapid Commun., 2009, 30, 925–931 CrossRef CAS PubMed.
- L. Ye, S. Zhang, W. Zhao, H. Yao and J. Hou, Chem. Mater., 2014, 26, 3603–3605 CrossRef CAS.
- D. Ouyang, M. Xiao, D. Zhu, W. Zhu, Z. Du, N. Wang, Y. Zhou, X. Bao and R. Yang, Polym. Chem., 2015, 6, 55–63 RSC.
- D. Lee, E. Hubijar, G. J. D. Kalaw and J. P. Ferraris, Chem. Mater., 2012, 24, 2534–2540 CrossRef CAS.
- D. Lee, S. W. Stone and J. P. Ferraris, Chem. Commun., 2011, 47, 10987–10989 RSC.
- M.-J. Baek, S.-H. Lee, K. Zong and Y.-S. Lee, Synth. Met., 2010, 160, 1197–1203 CrossRef CAS PubMed.
- J. H. Park, Y. G. Seo, D. H. Yoon, Y.-S. Lee, S.-H. Lee, M. Pyo and K. Zong, Eur. Polym. J., 2010, 46, 1790–1795 CrossRef CAS PubMed.
- J. Zhang, W. Cai, F. Huang, E. Wang, C. Zhong, S. Liu, M. Wang, C. Duan, T. Yang and Y. Cao, Macromolecules, 2011, 44, 894–901 CrossRef CAS.
- E. Zhou, J. Cong, K. Tajima and K. Hashimoto, Chem. Mater., 2010, 22, 4890–4895 CrossRef CAS.
- Y. Li, Y. Cao, J. Gao, D. Wang, G. Yu and A. J. Heeger, Synth. Met., 1999, 99, 243–248 CrossRef CAS.
- M. C. Scharber, D. Mühlbacher, M. Koppe, P. Denk, C. Waldauf, A. J. Heeger and C. J. Brabec, Adv. Mater., 2006, 18, 789–794 CrossRef CAS PubMed.
- M. Campoy-Quiles, T. Ferenczi, T. Agostinelli, P. G. Etchegoin, Y. Kim, T. D. Anthopoulos, P. N. Stavrinou, D. D. C. Bradley and J. Nelson, Nat. Mater., 2008, 7, 158–164 CrossRef CAS PubMed.
- M. Drees, H. Hoppe, C. Winder, H. Neugebauer, N. S. Sariciftci, W. Schwinger, F. Schäffler, C. Topf, M. C. Scharber, Z. Zhu and R. Gaudiana, J. Mater. Chem., 2005, 15, 5158 RSC.
| This journal is © The Royal Society of Chemistry 2015 |



