Review on nanomaterials synthesized by vapor transport method: growth and their related applications
Abstract
Nanostructures with different dimensions, including bulk crystals, thin films, nanowires, nanobelts and nanorods, have received considerable attention due to their novel functionalities and outstanding applications in various areas, such as optics, electricity, thermoelectricity, photovoltaic fields and sensing devices. In recent years, remarkable progresses and modifications have been made upon the fabrication of nanomaterials by vapor transport method. In this review, we introduce various representative nanostructures prepared by vapor transport method and focus on the discussion of their growth, physical properties, and potential applications. Meanwhile, the essential growth mechanisms of nanostructures also have been extensively reviewed, for example, the different growth modes depending upon the specific sample growth. Finally, we conclude this review by providing our perspectives to the future vapor transport method, and indicating some key existing problems. Vapor transport process offers great opportunities for the low-cost preparation of novel single crystals with different doping level and the realization of integrating such nano/micro single crystals into spintronic and electronic devices.
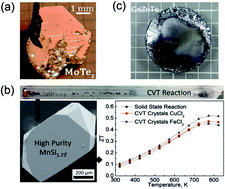

 Please wait while we load your content...
Please wait while we load your content...