Rational synthesis of zerovalent iron/bamboo charcoal composites with high saturation magnetization†
Abstract
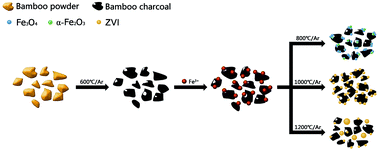
The synthesis of magnetic biochar composites is a major new research area in advanced materials sciences. A series of magnetic bamboo charcoal composites (MBC800, MBC1000 and MBC1200) with high saturation magnetization (Ms) was fabricated in this work by mixing bamboo charcoal powder with an aqueous ferric chloride solution and subsequently pyrolyzing under different temperatures (800, 1000 and 1200 °C) in a tube furnace. All the products were characterized using X-ray powder diffraction (XRD), X-ray photoelectron spectroscopy (XPS), field emission scanning electron microscopy (FESEM), energy dispersive X-ray spectrometer (EDAX), high-resolution transmission electron microscopy (HRTEM), Raman spectroscopy and a superconducting quantum interference device (SQUID). The results show that the pyrolytic temperature plays a significant role in determining the final structures and magnetic properties of the products. The magnetite (Fe3O4) and a certain amount of amorphous hematite (α-Fe2O3) coexist in MBC800. However, zerovalent iron (ZVI) is the only detectable magnetic phase in both MBC1000 and MBC1200. The Ms values of MBC1000 and MBC1200 are 118.1 and 122.7 emu g−1, respectively. Excellent magnetic properties of the two ZVI/bamboo charcoal composites not only facilitate the separation of solid phase, but also indicate that these materials could have high potential for other applications, such as in the biomedical or ferrofluid fields.

 Please wait while we load your content...
Please wait while we load your content...