Influence of hyperbranched copper phthalocyanine grafted carbon nanotubes on the dielectric and rheological properties of polyarylene ether nitriles
Zejun Pu,
Lifen Tong,
Mengna Feng,
Kun Jia* and
Xiaobo Liu*
Research Branch of Advanced Functional Materials, Institute of Microelectronic & Solid State Electronic, High-Temperature Resistant Polymers and Composites Key Laboratory of Sichuan Province, University of Electronic Science & Technology of China, Chengdu 610054, P. R. China. E-mail: liuxb@uestc.edu.cn; Jiakun@uestc.edu.cn; Fax: +86-028-83207326; Tel: +86-028-83207326
First published on 18th August 2015
Abstract
Novel hyperbranched copper phthalocyanine covalently grafted carbon nanotube/polyarylene ether nitrile (HBCuPc-CNT/PAEN) flexile composite films were prepared via solution casting. The CNTs are enwrapped by a functional intermediate HBCuPc thin layer which forms a rough shell on the surface of the CNTs to ensure a good dispersion of CNTs in the PAEN matrix. The dielectric layer (HBCuPc-CNTs) is intercalated by insulating layers (pure PAEN, acting as the isolating layer). Due to the high capacitance of the dielectric layer and the effective blocking of the mobility of free charge carriers by the insulating layers, the polymer-based composite films exhibit not only a high permittivity but also an extremely low dielectric loss and excellent breakdown strength. SEM images show that HBCuPc-CNTs are perfectly embedded in the matrix and no pull-out phenomenon can be observed. In addition, the rheological properties of the resulting composite films also indicate that the grafted CNTs present a good dispersion and strong interactions with the PAEN resin, thus resulting in a significant improvement of the mechanical and thermal properties of the PAEN composite films.
Introduction
Materials with high permittivity (called high-k dielectric materials) and low dielectric loss (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) have been extensively researched in recent decades for their application in capacitors, actuators and high energy density pulsed power.1–4 Among those dielectric materials, metals and ceramics with excellent thermal stability and high stiffness have also a high k, but they do not meet the requirements for some applications well because of their high density, brittleness and challenging processing conditions. To avoid these problems, polymeric dielectric materials have received special attention for their excellent properties (e.g., high electric breakdown field, low dielectric loss, flexibility, easy processing and low cost, etc.). Polymer and polymer-based composite dielectrics offer an attractive alternative to conventional ceramic dielectrics in many fields, but most polymers have often low intrinsic permittivity, in the range of 2–5,5 impeding their use for high-k applications. An effective method to enhance the permittivity of a polymer is through the construction of polymer-based composites with prominent dielectric properties, while retaining other excellent properties.6–8 In addition, their good adhesive properties are an additional advantage for their use in embedded capacitor technologies, which pure ceramics or other dielectric materials lack.
δ) have been extensively researched in recent decades for their application in capacitors, actuators and high energy density pulsed power.1–4 Among those dielectric materials, metals and ceramics with excellent thermal stability and high stiffness have also a high k, but they do not meet the requirements for some applications well because of their high density, brittleness and challenging processing conditions. To avoid these problems, polymeric dielectric materials have received special attention for their excellent properties (e.g., high electric breakdown field, low dielectric loss, flexibility, easy processing and low cost, etc.). Polymer and polymer-based composite dielectrics offer an attractive alternative to conventional ceramic dielectrics in many fields, but most polymers have often low intrinsic permittivity, in the range of 2–5,5 impeding their use for high-k applications. An effective method to enhance the permittivity of a polymer is through the construction of polymer-based composites with prominent dielectric properties, while retaining other excellent properties.6–8 In addition, their good adhesive properties are an additional advantage for their use in embedded capacitor technologies, which pure ceramics or other dielectric materials lack.
As a milestone, carbon nanotubes (CNTs) are well known for their intriguing properties such as mechanical, thermal and electrical properties.9–11 Particularly, their unique structural, thermal and mechanical properties make them an ideal reinforcing material for polymer-based composites. Besides, the superior electrical properties of CNTs offer exciting opportunities for new high-k polymer-based composites. In addition, their high surface area and large aspect ratio are also responsible for the excellent dielectric properties of CNT/polymer composites.12,13 Good dispersion and interfacial interactions are two key issues in order to achieve the reinforcement of polymer composites.14 The large aspect ratio and poor dispersion of CNTs cause them to become easily agglomerated, which ultimately causes the direct contact between the dispersed conductive fillers, leading to high tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ or even conduction at or above the percolation threshold. Thus, various strategies have been employed, including non-covalent adsorption, wrapping of various functional molecules and covalent attachment of chemical groups. Among these strategies, surface modification via covalent chemistry is the preferred method.15 Polyarylene ether nitrile (PAEN), as a special thermoplastic engineering material, possesses various outstanding properties, including excellent mechanical properties, high thermal stability, low flammability, radiation resistance and chemical inertness, etc. These characteristics make it a good candidate for usage in industrial, automotive and outer space fields, which involve high temperatures or high radiation exposure.16
δ or even conduction at or above the percolation threshold. Thus, various strategies have been employed, including non-covalent adsorption, wrapping of various functional molecules and covalent attachment of chemical groups. Among these strategies, surface modification via covalent chemistry is the preferred method.15 Polyarylene ether nitrile (PAEN), as a special thermoplastic engineering material, possesses various outstanding properties, including excellent mechanical properties, high thermal stability, low flammability, radiation resistance and chemical inertness, etc. These characteristics make it a good candidate for usage in industrial, automotive and outer space fields, which involve high temperatures or high radiation exposure.16
Copper phthalocyanine (CuPc) oligomers, as organic semiconductors, possess a good dielectric response because of the long-range intermolecular hopping of electrons, and their permittivity can reach up to 106.17 In addition, hyperbranched copper phthalocyanines (HBCuPcs) with dendritic molecular structure provide more charge carriers and a large carrier mobility, which are responsible for a high dielectric response.18 In this study, we report the fabrication of novel HBCuPc-CNTs via a simple and effective covalent bonding method. Then, a series of HBCuPc-CNT/PAEN composite films were prepared by a solution casting method. The functional intermediate HBCuPc thin layer grafted onto the surface of the CNTs improves the dispersion and interfacial interactions of the CNTs in the PAEN matrix, which decreases the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ of the resulting composite films. Thus, the composites display remarkably improved dielectric properties and energy storage density compared to pure PAEN. The combined influence of the HBCuPc-CNTs on the other properties of the composite films has also been investigated.
δ of the resulting composite films. Thus, the composites display remarkably improved dielectric properties and energy storage density compared to pure PAEN. The combined influence of the HBCuPc-CNTs on the other properties of the composite films has also been investigated.
Experimental
Materials
CNTs (purity: >95%, diameter: about 20 nm, length: 30–50 μm) were purchased from Chengdu Organic Chemicals Co., Ltd, Chinese Academy of Science. After being sonicated and refluxed in a H2SO4–HNO3 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume) mixture of solvents, acidulated CNTs (a-CNTs) with abundant –COOH and –OH groups were obtained. Copper(I) chloride (CuCl), N-methylpyrrolidone (NMP, AR), N,N-dimethylformamide (DMF, AR) and N,N-dimethylacetamide (DMAc, AR) were supplied by Tianjin BODI chemicals. Isophorone diisocyanate (IPDI) was supplied by Shanghai Ye Hing Industrial Co., Ltd. Polyarylene ether nitrile, 4-aminophenoxyphthalonitrile (4-APN) and 1,3,5-tri-(3,4-dicyanophenoxy) benzene (TPh) were prepared in our laboratory.
1 by volume) mixture of solvents, acidulated CNTs (a-CNTs) with abundant –COOH and –OH groups were obtained. Copper(I) chloride (CuCl), N-methylpyrrolidone (NMP, AR), N,N-dimethylformamide (DMF, AR) and N,N-dimethylacetamide (DMAc, AR) were supplied by Tianjin BODI chemicals. Isophorone diisocyanate (IPDI) was supplied by Shanghai Ye Hing Industrial Co., Ltd. Polyarylene ether nitrile, 4-aminophenoxyphthalonitrile (4-APN) and 1,3,5-tri-(3,4-dicyanophenoxy) benzene (TPh) were prepared in our laboratory.
Preparation of HBCuPc-CNT and PAEN composite films
IPDI possesses two isocyanate groups with different activities. Thus, nitrile functionalized CNTs (CNTs-CN) were prepared by a two-step synthesis in which the hydroxyl and carboxyl groups on the CNTs firstly reacted with the secondary isocyanate group of IPDI to get CNTs-IPDI. Then, the primary isocyanate group of the IPDI grafted onto the CNTs reacted with the amino group of 4-APN. Thus, CNTs-CN were obtained and the detailed process is as follows: 0.5 g of a-CNTs, 5 mL IPDI and 35 mL DMF were added to a 100 mL round-bottom flask under a nitrogen atmosphere. Then, the mixture was refluxed with magnetic stirring at 50 °C for 7 h. The excess IPDI was removed by filtration, leaving a blackish block. Then, the remaining solid was reacted with 4-aminophenoxyphthalonitrile (4-APN) (0.4 g) in 40 mL DMF at 85 °C for 5 h. The excess 4-APN was totally removed by washing with dichloromethane and acetone twice, respectively. After drying under vacuum at 50 °C overnight, CNTs-CN was obtained, which was subsequently used for in situ polymerization in the presence of TPH and CuCl at 160 °C for 4 h, thereby yielding HBCuPc-grafted CNTs. The detailed experimental description is shown in Fig. 1. Meanwhile, a series of HBCuPc-CNT/PAEN and a-CNT/PAEN composite films were prepared through the solution casting method according to ref. 16. The mass fraction of the fillers in the PAEN matrix was fixed at 0%, 1.0%, 2.0%, 3.0%, 5.0%, 7.0%, 9.0%. Thus, composite films of about 60 ± 20 μm in thickness were obtained.Characterization
The synthesized products were characterized by scanning electron microscopy (SEM, JSM-5900LV), transmission electron microscopy (TEM, Hitach H600) and Fourier transform infrared spectroscopy (FTIR, 200SXV) in KBr pellets. Ultraviolet-visible (UV-Vis) absorption spectra were recorded with a Shimadzu 3100 UV-Vis-near-IR spectrophotometer. The cross section microstructure of the films was observed by SEM. Dynamical rheological measurements were carried out using a rheometer (Rheometer AR-G2) equipped with parallel-plate geometry (25 mm diameter). The mechanical properties of the films were studied with a SANS CMT6104 Series Desktop Electromechanical Universal Testing Machine (stretching speed: 5 mm min−1). Differential scanning calorimetry (DSC) analysis of the films was carried out on a TA Instrument DSC Q100 under nitrogen atmosphere from 50 °C to 350 °C at a heating rate of 10 °C min−1. Thermogravimetric analysis (TGA) of various CNTs and the composite films was carried out under N2 atmosphere from 50 °C to 800 °C at a heating rate of 20 °C min−1. The dielectric properties of the films were monitored according to ASTM D150 on a TH 2819A precision LCR meter. The composite films were cut into small circular disks (diameter: 10 mm), and the thickness of each sample was measured too. Then, conductive silver paste was brushed onto a specific area on both sides of the sample to form a plate capacitor. Moreover, the dielectric experiments were carried out in the frequency range of 50 Hz to 200 kHz at room temperature. The breakdown strength tests were conducted by a DC dielectric strength tester with a sphere–sphere copper electrode (ZJC-100 kV) at room temperature. The samples were tested in an oil bath. The applied voltage began at 40 V (DC) and increased at approximately 0.1 kV s−1 until the breakdown of a sudden current increased. The breakdown tests were performed on six specimens of each sample with a thickness of about 60 ± 20 μm.Results and discussion
Characterization of HBCuPc-CNTs
The morphology of a-CNTs and HBCuPc-CNTs was investigated by SEM and TEM, respectively. It can be seen from Fig. 2a that the a-CNTs present a smooth surface and loosely packed arrangement, while HBCuPc-CNTs present obvious changes in the morphology and structure in comparison to a-CNTs. As seen from Fig. 2b, the surface of HBCuPc-CNTs is rougher and the average diameter is also bigger than those of a-CNTs. Moreover, the image of HBCuPc-CNTs also shows superposed spherical particles of HBCuPc wrapped around the surface of the CNTs, resulting from the cyclization reaction of polyfunctional phthalonitrile (TPH). Furthermore, the microstructure of the a-CNTs and HBCuPc-CNTs was investigated by TEM as well. It is clear that the a-CNTs have a smooth and clean surface without any extra phase adhered to and between them (Fig. 2c). In contrast, the TEM image of HBCuPc-CNTs shows that the CNTs are enwrapped by a functional intermediate HBCuPc thick layer (Fig. 2d). It should be noted that the entire surface of the CNTs was modified with HBCuPc, which formed a rough shell on the surface of the CNTs, indicating a strong interaction between HBCuPc and the CNTs.TGA and DTG analyses were used to study the composition of different CNTs. As shown in Fig. 3a, the weight of raw CNTs almost remains the same up to a temperature of 800 °C. The a-CNTs present a weight loss of 8.7 wt%. However, the DTG analysis (from the inset of Fig. 3a) indicates that the CNTs-CN and HBCuPc-CNTs have two noticeable increases in weight loss in the temperature ranges of 200–350 °C and 350–500 °C, which could be due to the pyrolysis of 4-APN and phthalocyanine, respectively. Thus, the content of the phthalocyanine covalently bonded to the surface of CNTs was calculated to be about 45.9 wt%, based on the total weight of the HBCuPc-CNTs according to the TGA and DTG analyses.
 | ||
| Fig. 3 (a) TGA and DTG (inset) curves of different CNTs; (b) FTIR and (c) UV-Vis spectra of various CNTs; (d) XPS fully scanned spectrum of HBCuPc-CNTs. | ||
The nature of the chemical groups on the surface of the CNTs was investigated by FTIR spectroscopy as shown in Fig. 3b. The absorption bands of a-CNTs at around 3420 and 1725 cm−1 are ascribed to hydroxyl and carboxyl groups, respectively. After treatment with IPDI and 4-APN, the bands at 1725 cm−1 disappear, followed by the appearance of new bands at 1697 cm−1 (the carbonyl stretching vibration of the carbamate esters), 1588 cm−1 (an amide carbonyl-stretching mode) and 1245 cm−1 (aromatic ether stretching vibration). Moreover, the absorption band at 2231 cm−1 belongs to the symmetrical stretching vibration of nitrile groups (–CN).16 All these observations indicate that 4-APN has been successfully grafted onto the surface of the CNTs. For HBCuPc-CNTs, the absorption peak intensity of –CN is weakened. More importantly, numerous characteristic absorption peaks are observed at 1605 cm−1, 1123 cm−1, 1089 cm−1, 905 cm−1 and 751 cm−1, which indicate the formation of a phthalocyanine ring. These results are well in agreement with the UV-Vis analysis, as shown in Fig. 3c. HBCuPc and HBCuPc-CNTs have two strong absorption bands in both visible and ultraviolet regions compared to those of a-CNTs and CNTs-CN. HBCuPc-CNTs exhibit a strong Q-band absorption peak in the wavelength range of 600–700 nm, which is attributed to π–π* transitions on the phthalocyanine macrocycle. The absorption peak at wavelengths of 300–400 nm belongs to the typical Soret band (B-band) absorption of the phthalocyanine ring.19 Therefore, the surface functionalization of CNTs was successfully realized by the cyclization reaction between CNTs-CN and TPH.
XPS was employed to further characterize the functionalization in detail. As can be seen in Fig. 3d, the full spectrum demonstrates that Cu, O, N, and C elements are present in HBCuPc-CNTs, revealing that the synthesized products are composed of the copper phthalocyanine derivative and CNTs. Additionally, three obvious peaks are observed at 287 eV, 403 eV and 534.5 eV, corresponding to C 1s, N 1s and O 1s, respectively. Furthermore, for the HBCuPc-CNTs sample, there are two slight symmetric peaks in the Cu 2p region. The peak centered at 933.8 eV corresponds to the Cu 2p3/2 and the other at 954 eV is assigned to Cu 2p1/2, indicating a normal Cu2+ state in HBCuPc-CNTs.
Dispersion of HBCuPc-CNTs in the PAEN matrix
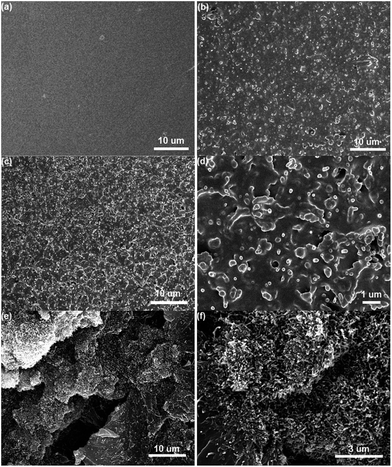
To develop high performance polymer/CNT composite films, it is very important to improve the dispersion and adhesion of CNTs in the polymer matrix.20 Fig. 4 shows the microstructures of pure PAEN and PAEN composite films. As can be seen, pure PAEN exhibits a relatively smooth and homogeneous fracture surface (Fig. 4a). However, the incorporation of HBCuPc-CNTs and a-CNTs significantly changes the morphology of the fracture surface. Although the same amount (5.0 wt%) of HBCuPc-CNTs and a-CNTs are present in the PAEN composite films, HBCuPc-CNTs are homogeneously dispersed and embedded in the PAEN matrix perfectly (Fig. 4b). When the content of the filler reaches up to 9.0 wt%, the PAEN matrix content is not enough to fill the gaps between the HBCuPc-CNTs. The high mass fraction of the HBCuPc-CNTs in the composite films may result in an increase in the porosity, and the HBCuPc-CNTs tend to self-aggregate (as seen from Fig. 4c). However, the enlarged detail of Fig. 4c (Fig. 4d) shows that the HBCuPc-CNTs are well embedded in the PAEN composite film. Moreover, all of the HBCuPc-CNTs are tightly held to the PAEN polymer, which can prevent the sliding of CNTs during tension. In comparison, the dispersion state of a-CNTs in the PAEN matrix is not as good and self-aggregation is obvious (Fig. 4e). From the enlarged image in Fig. 4f, the phenomenon of “pull-out” is clearly observed in the a-CNT/PAEN composite film. These results can be attributed to the benefit of the functionalized –CN groups, promoting the adhesion between HBCuPc-CNTs and the PAEN matrix. Consequently, the introduction of the functional intermediate HBCuPc thick layer onto CNTs can effectively improve the interface between the CNTs and the PAEN matrix.Linear viscoelastic behavior of the composite films
Generally, melt rheology is of basic and practical importance since it is related to the composite’s processing, dynamics, structure–property relationships and the interaction between fillers and the polymer matrix in composite systems.21,22 Fig. 5 presents the complex viscosity (η*) of the composite films. In Fig. 5a, the η* of pure PAEN shows little frequency dependence and almost behaves like a Newtonian fluid, since the polymer chain is fully relaxed at all frequencies. As the filler content increases, the slope of the low-frequency η* slightly increases and the Newtonian plateau of the viscosity curve disappears gradually. A dramatic increase happens when HBCuPc-CNTs is over 3 wt% and the effect of HBCuPc-CNTs on the η* of the PAEN composite film is more significant at low frequencies compared to high frequencies (Fig. 5b). Thus, the sudden η* change at low frequencies indicates a transition from a viscoelastic liquid- to a solid-like behavior. This behavior transition is attributed to the formation of a percolated structure by the fillers.21,22 | ||
| Fig. 5 Complex viscosity (η*) of (a) PAEN/HBCuPc-CNTs composite films and (b) complex viscosity (η*) as a function of filler loading. | ||
The storage modulus (G′) represents the elastic melt properties and provides a measure of a composite’s ‘stiffness’, while the loss modulus (G′′) stands for viscous melt properties and reveals information on ‘softness’. Their frequency dependence characterizes whether the material is in a solid-like or liquid-like state, which can further provide information on the percolated structure. Thus, Fig. 6 presents the G′ and G′′ values resulting from dynamic frequency scan measurements. The magnitude of G′ monotonously increases with the increasing oscillatory frequency (Fig. 6a). For PAEN composite films with different HBCuPc-CNT loadings, the increment of G′ is more obvious, especially at low frequencies, which is attributed to the reinforcing effect of the CNTs. Clearly, at low frequencies, the PAEN composite films with loadings below 5.0 wt% HBCuPc-CNTs show almost the same slope of G′ versus frequency as that of pure PAEN in the terminal region. That is, the PAEN chains are fully relaxed and present typical near-terminal behavior with scaling properties of about G′ ∝ ω2, which is consistent with the Cox–Merz rule.16 With a larger content of HBCuPc-CNTs, however, the dependence with the low frequency weakens and the slope of G′ becomes flat, indicating a pseudo-solid like behavior. This phenomenon has also been observed in both polymer/CNT and polymer/graphene systems, which is mainly due to the existence of interactions between fillers and the polymer matrix.21,22 The same trend is observed in Fig. 6b for the G′′ curves. These results reveal that the large scale polymer relaxation is effectively restrained by the presence of HBCuPc-CNTs. Fig. 6c shows the relationship between G′ and HBCuPc-CNT loading for the PAEN composite films at 0.1 rad s−1. Clearly, G′ increases abruptly between 3.0 and 5.0 wt% loadings, revealing that there is a sudden change in the material phase structure. This also confirms that a rheological percolation network has been formed at about 4.0 wt% HBCuPc-CNTs loading and that the HBCuPc-CNTs effectively restrain the relaxation process in the region of the molten state of PAEN chains to some extent. These restrictions may be described as physical entanglement and chemical interfacial interactions.23,24 In this case, the superposition-type spherical compound-like HBCuPc on the surface of the CNTs can increase the knotting of molecules and interlock with the PAEN matrix.25 Besides, the strongly polar CN groups on the functionalized CNTs can also interact with the PAEN matrix.
Mechanical and thermal properties of the composite films
Carbon nanotubes are considered as promising reinforcing materials for polymer-based composites which can improve the mechanical and thermal properties of polymers.26,27 Fig. 7 shows the influence of HBCuPc-CNTs on the mechanical properties of PAEN composite films. Significantly, for the 2.0 wt% HBCuPc-CNTs reinforced PAEN composite film (Fig. 7a), the tensile strength and modulus of HBCuPc-CNT/PAEN (117 and 2729 MPa) reach a higher value in comparison with those of pure PAEN (92 and 2244 MPa). With further increase of the HBCuPc-CNTs loading, the tensile strength and modulus decrease gradually, but almost remain higher than those of pure PAEN. The dispersion of HBCuPc-CNTs restricts the mobility of the PAEN chains, which improves the tensile modulus and the strength at low loading. In addition, the high aspect ratio, high modulus and strength of the CNTs also contribute to the reinforcement. With the increase of the HBCuPc-CNTs loading, the decrease in the elongation may be ascribed to the self-aggregation of the HBCuPc-CNTs and some microvoids formed in the PAEN matrix (Fig. 7b). Besides, the photographs in Fig. 7c demonstrate the high degree of flexibility of the PAEN composite films. These results indicate that the mechanical performance of the composite films was strengthened with the addition of HBCuPc-CNTs.DSC and TGA analyses were used to study the effect of HBCuPc-CNTs on the thermal properties of the composite films and the detailed data are summarized in Table 1, in which the glass transition temperature (Tg), the decomposition temperature at weight loss 5% (T5%) and the temperature corresponding to the maximum rate of decomposition (Tmax) are listed. It can be seen in Table 1 that all the composite films show a higher Tg in comparison with pure PAEN. For 9.0 wt% HBCuPc-CNT reinforced PAEN composite films, the Tg increased by 8 °C. This is due to the improved dispersibility of CNTs and the strong interaction between the functional intermediate HBCuPc and the PAEN matrix, which can reduce the free volume and segmental mobility, therefore improving Tg. The TGA analysis shows that there are no obvious changes in the thermal stability of the composite films with the increase of the HBCuPc-CNT loading: the T5% and Tmax of the composite films are as high as those of the pure PAEN film. In conclusion, these results may be ascribed to the special thermal stability of the CNTs and the PAEN matrix.
| HBCuPc-CNTs content (wt%) | 0.0 | 1.0 | 2.0 | 3.0 | 5.0 | 7.0 | 9.0 |
|---|---|---|---|---|---|---|---|
| Tg (°C) | 200 | 206 | 207 | 208 | 207 | 209 | 208 |
| T5% (°C) | 522 | 519 | 518 | 525 | 516 | 527 | 524 |
| Tmax (°C) | 539 | 538 | 534 | 541 | 535 | 547 | 540 |
| Cy (%) | 63.5 | 66.3 | 67.6 | 67.9 | 68.0 | 68.5 | 69.0 |
Dielectric properties of the composite films
Fig. 8a and b show the changes in the dielectric constant (ε) and dielectric loss (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ), respectively. As can be seen, the dielectric properties of all composite films showed little frequency dependence at all given frequencies. At high HBCuPc-CNT loadings (>3.0 wt%), ε decreases gradually with the increasing frequency, whereas it remains as high as about 10 even at a frequency of 200 kHz. Moreover, tan
δ), respectively. As can be seen, the dielectric properties of all composite films showed little frequency dependence at all given frequencies. At high HBCuPc-CNT loadings (>3.0 wt%), ε decreases gradually with the increasing frequency, whereas it remains as high as about 10 even at a frequency of 200 kHz. Moreover, tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ of all the composite films also exhibits a slight frequency dependence at low frequencies but they are independent at high frequencies. These variations are consistent with the results in the literature for PAEN/HBCuPc composites.28 Fig. 8c shows the effect of HBCuPc-CNT loading on the dielectric behavior of all the composite films. As expected, the ε of all composite films gradually rises with the increasing HBCuPc-CNT loading. Typically, the ε of the PAEN composite film with 9.0 wt% HBCuPc-CNT content displays a 13-fold increase at 1 kHz (up to 51.7 from 3.9 of the pure PAEN), whereas the tan
δ of all the composite films also exhibits a slight frequency dependence at low frequencies but they are independent at high frequencies. These variations are consistent with the results in the literature for PAEN/HBCuPc composites.28 Fig. 8c shows the effect of HBCuPc-CNT loading on the dielectric behavior of all the composite films. As expected, the ε of all composite films gradually rises with the increasing HBCuPc-CNT loading. Typically, the ε of the PAEN composite film with 9.0 wt% HBCuPc-CNT content displays a 13-fold increase at 1 kHz (up to 51.7 from 3.9 of the pure PAEN), whereas the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ is as low as 0.23. Compared to PAEN-based composite films, even with high-k fillers and high loadings, the ε of the 9.0 wt% HBCuPc-CNT reinforced PAEN composite film at 100 Hz is about 3-fold higher than that of the CNTs-CN/PAEN composite film (with 8.0 wt% CNTs-CN)29 and 24-fold higher than that of HBCuPc/PAEN (with 30 wt% HBCuPc).28
δ is as low as 0.23. Compared to PAEN-based composite films, even with high-k fillers and high loadings, the ε of the 9.0 wt% HBCuPc-CNT reinforced PAEN composite film at 100 Hz is about 3-fold higher than that of the CNTs-CN/PAEN composite film (with 8.0 wt% CNTs-CN)29 and 24-fold higher than that of HBCuPc/PAEN (with 30 wt% HBCuPc).28
In fact, the increase of ε in composite films has been widely reported and may also be understood through a micro-capacitor network model.1,30 HBCuPc was grafted onto CNTs, which effectively improved the dielectric properties of the HBCuPc-CNT/PAEN composite films. The effect is attributed to the HBCuPc grafting, which gives a better dispersion of CNTs in the PAEN matrix and therefore, lots of conductive CNTs are isolated by dielectric insulating-polymer layers within the composite films, forming lots of micro-capacitors. As the HBCuPc-CNT content increases, the expected number of micro-capacitors increases too, resulting in a high capacitance and thus a high ε. Moreover, HBCuPc grafting effectively suppress the mobility of free charge carriers, and the charge is also increased by introducing the strong polar nitrile groups and phthalocyanine rings on the surface of CNTs. Consequently, the ε of the composite films is increased with increasing HBCuPc-CNT loadings. Meanwhile, the low tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ of all composite films can be attributed to a better dispersion of the HBCuPc-CNTs and the strong interfacial interactions between the CNTs and the PAEN matrix. Therefore, HBCuPc grafted CNTs are an efficient and effective system to improve the dielectric properties of PAEN composite films.
δ of all composite films can be attributed to a better dispersion of the HBCuPc-CNTs and the strong interfacial interactions between the CNTs and the PAEN matrix. Therefore, HBCuPc grafted CNTs are an efficient and effective system to improve the dielectric properties of PAEN composite films.
For a dielectric material, a high permittivity is not the only factor that governs its energy storage capability, a high breakdown strength (Eb) is also important for large energy storage capability, since it determines the operating electric field and the energy storage density of the dielectric material.31 The permittivity and breakdown strength of the films as a function of the HBCuPc-CNT loading measured at room temperature are listed in Table 2. The energy density was calculated from eqn (1) and is presented in Fig. 8d. The energy density (U) of a capacitor is given by the following equation:
 | (1) |
| HBCuPc-CNTs content (wt%) | 0.0 | 1.0 | 2.0 | 3.0 | 5.0 | 7.0 | 9.0 |
|---|---|---|---|---|---|---|---|
| Dielectric constant, εr (at 1 kHz) | 3.7 | 5.3 | 7.4 | 12.3 | 19.7 | 24.9 | 51.7 |
| Breakdown strength, Eb (kV mm−1) | 180 | 154 | 144.8 | 127.5 | 120 | 115 | 112 |
| Energy density, U (J cm−3) | 0.53 | 0.57 | 0.69 | 0.89 | 1.26 | 1.46 | 2.87 |
Conclusions
In conclusion, we have presented an effective method to covalently graft a dielectric layer (HBCuPc), containing strong polar nitrile groups and phthalocyanine rings, onto the surface of CNTs, which obviously improved their dispersion in PAEN composites. Consequently, the HBCuPc-CNTs had a significant enhancement effect on the mechanical and thermal properties of PAEN composites. Meanwhile, the loading amount and existence of the phthalocyanine phase within the composites also had an influence on the dielectric properties of the composites. Typically, the permittivity of the PAEN composite film with 9.0 wt% HBCuPc-CNT reached 117 F m−1 at 100 Hz, whereas the dielectric loss tangent was as low as 0.41. More importantly, the composite films show a high breakdown strength and large energy storage density, which are desirable qualities for capacitors for energy storage. Thus, the unique features of the PAEN composites make them potential high performance dielectric materials for application in the capacitor field.Acknowledgements
The authors wish to thank for the financial support of this work the National Natural Science Foundation (No. 51173021, 51373028, 51403029), “863” National Major Program of High Technology (2012AA03A212), Ningbo Major (key) Science and Technology Research Plan (2013B06011) and South Wisdom Valley Innovative Research Team Program.Notes and references
- Z. M. Dang, J. K. Yuan, J. W. Zha, T. Zhou, S. T. Li and G. H. Hu, Prog. Mater. Sci., 2012, 57, 660–723 CrossRef CAS PubMed.
- X. F. Li, W. H. Xu, Y. H. Zhang, D. Xu, G. B. Wang and Z. H. Jiang, RSC Adv., 2015, 5, 51542–51548 RSC.
- Z. H. Zhou, J. M. Xue, W. Z. Li, J. Wang, H. Zhu and J. M. Miao, Appl. Phys. Lett., 2004, 85, 804 CrossRef CAS PubMed.
- Q. Li, Q. G. Xue, L. Z. Hao, X. L. Gao and Q. B. Zheng, Compos. Sci. Technol., 2008, 68, 2290–2296 CrossRef CAS PubMed.
- R. H. Baughman, A. A. Zakhidov and W. A. Heer, Science, 2002, 297, 787–792 CrossRef CAS PubMed.
- Q. M. Zhang, H. Li, M. Poh, F. Xia, Z. Y. Cheng and C. Huang, Nature, 2002, 419, 284–287 CrossRef CAS PubMed.
- P. Brochu and Q. B. Pei, Macromol. Rapid Commun., 2010, 31, 10–36 CrossRef CAS PubMed.
- Z. M. Dang, S. H. Yao, J. K. Yuan and R. J. Liao, Adv. Mater., 2013, 25, 6334–6365 CrossRef CAS PubMed.
- E. W. Wong, P. E. Sheehan and C. M. Lieber, Science, 1997, 277, 1971–1975 CrossRef CAS.
- P. Miaudet, C. Bartholome, A. Derre, M. Maugey, G. Sigaud, C. Zakri and P. Poulin, Polymer, 2007, 48, 4068–4074 CrossRef CAS PubMed.
- R. H. Baughman, A. A. Zakhidov and W. A. de-Heer, Science, 2002, 297, 787–792 CrossRef CAS PubMed.
- Z. M. Dang, L. Wang, Y. Yin, Q. Zhang and Q. Q. Lei, Adv. Mater., 2007, 19, 852–857 CrossRef CAS PubMed.
- X. Huang, K. Wang, K. Jia and X. B. Liu, RSC Adv., 2015, 5, 51975–51982 RSC.
- K. Ke, Y. Wang, X. Q. Liu, J. Cao, Y. Luo, W. Yang, B. H. Xie and M. B. Yang, Composites, Part B, 2012, 43, 1425–1432 CrossRef CAS PubMed.
- T. J. Lua, M. Jiang, Z. G. Jiang, D. Hui and Z. W. Zhou, Composites, Part B, 2013, 51, 28–34 CrossRef PubMed.
- Z. J. Pu, H. L. Tang, X. Huang, J. Yang, Y. Q. Zhan, R. Zhao and X. B. Liu, Colloids Surf., A, 2012, 415, 125–133 CrossRef CAS PubMed.
- P. S. Vijayakumar and H. A. Pohl, J. Polym. Sci., Polym. Phys. Ed., 1984, 22, 1439–1451 CrossRef CAS PubMed.
- T. W. Lee, Y. Kwon, J. J. Park, L. Pu, T. Hayakawa and M. Kakimoto, Macromol. Rapid Commun., 2007, 28, 1657–1662 CrossRef CAS PubMed.
- F. B. Meng and X. B. Liu, RSC Adv., 2015, 5, 7018–7022 RSC.
- X. Q. Liu, S. Q. Shen, R. Wen, W. Yang, B. H. Xie and M. B. Yang, Composites, Part B, 2013, 53, 9–16 CrossRef CAS PubMed.
- P. Pötschke, T. D. Fornes and D. R. Paul, Polymer, 2002, 43, 3247–3255 CrossRef.
- X. L. Yang, Y. Q. Zhan, R. Zhao and X. B. Liu, J. Appl. Polym. Sci., 2012, 124, 1723–1730 CrossRef CAS PubMed.
- Z. J. Pu, X. F. Zhou, X. L. Yang, K. Jia and X. B. Liu, J. Magn. Magn. Mater., 2015, 385, 368–376 CrossRef CAS PubMed.
- H. L. Tang, Z. Ma, J. C. Zhong, J. Yang, R. Zhao and X. B. Liu, Colloids Surf., A, 2011, 384, 311–317 CrossRef CAS PubMed.
- H. Guo, Y. Q. Zhan, Z. Chen, F. B. Meng, J. J. Wei and X. B. Liu, J. Mater. Chem. A, 2013, 1, 2286–2296 CAS.
- H. S. Kim, B. H. Park, J. S. Yoon and H. J. Jin, Eur. Polym. J., 2007, 43, 1729–1735 CrossRef CAS PubMed.
- M. Moniruzzaman and K. I. Winey, Macromolecules, 2006, 39, 5194–5205 CrossRef CAS.
- J. C. Zhong, H. L. Tang, Y. W. Chen and X. B. Liu, J. Mater. Sci.: Mater. Electron., 2010, 21, 1244–1248 CrossRef CAS.
- Z. J. Pu, X. Huang, L. Chen, J. Yang and X. B. Liu, J. Mater. Sci.: Mater. Electron., 2013, 24, 2913–2922 CrossRef CAS.
- F. He, S. Lau, H. L. Chan and J. Fan, Adv. Mater., 2009, 21, 710–715 CrossRef CAS PubMed.
- Y. Q. Chen, B. P. Lin, X. Q. Zhang, J. C. Wang, C. W. Lai, Y. Sun, Y. R. Liu and H. Yang, J. Mater. Chem. A, 2014, 2, 14118–14126 CAS.
| This journal is © The Royal Society of Chemistry 2015 |