Pyrene terminal functionalized perylene diimide as non-fullerene acceptors for bulk heterojunction solar cells
Xin Liu,
Guoping Luo,
Xinyi Cai,
Hongbin Wu,
Shi-Jian Su* and
Yong Cao
State Key Laboratory of Luminescent Materials and Devices, Institute of Polymer Optoelectronic Materials and Devices, South China University of Technology, Guangzhou 510640, P. R. China. E-mail: mssjsu@scut.edu.cn; Fax: +86 2087110606; Tel: +86 2022237098
First published on 24th September 2015
Abstract
Two perylene diimide (PDI) based small molecules with different terminal groups of pyrene and tert-butyl pyrene, namely P1 and P2, respectively, were designed and synthesized as the acceptor materials in organic solar cells (OSCs). The impacts of the different terminal groups combined with the PDI core on the optical absorption and fluorescence, electrochemical properties, film morphology, and solar cell performance were studied thoroughly. The two compounds possess a broad absorption covering the wavelength range of 400–650 nm and a relatively high LUMO energy level of 3.77 eV. Power conversion efficiency (PCE) of the OSCs based on P2 as the acceptor material and PTB7 as the donor material (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) is 0.41%. In contrast, a PCE of 1.35% was achieved for the device based on P1 as the acceptor and PTB7 as the donor (1
1, w/w) is 0.41%. In contrast, a PCE of 1.35% was achieved for the device based on P1 as the acceptor and PTB7 as the donor (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w).
1, w/w).
Introduction
Conventional bulk-heterojunction (BHJ) organic solar cells (OSCs) are based on polymer or small molecule materials as electron donors and fullerene derivatives as electron acceptors.1–5 Compared with inorganic solar cells, OSCs are made with non-toxic and cheap materials and are manufactured by low cost technologies such as solution processes or a roll-to-roll process on large areas of light-weight flexible substrates.6,7 The performance of the fullerene based OSCs has also been improving steadily with power conversion efficiencies (PCEs) surpassing 10% for single junction OSCs.8–10 Despite the essential role of fullerenes in achieving best performance OSCs, fullerene acceptors have several drawbacks including poor light absorption, high-cost production and purification.11,12 For this reason, OSCs based on small molecular non-fullerene acceptors have attracted much attention due to the easy adjustment of electronic and optical properties of non-fullerene acceptor materials and have been improving progressively during the past few years. There have been many reports of non-fullerene-based OSCs achieving PCEs ranging from 3–5% until a recent report of PCE passing 6%.13–25The development of alternative acceptor materials that exhibit favourable electron transporting and good sunlight absorption is very important. Perylene diimides (PDIs) are suitable candidates as acceptors in OSCs, although PDI molecules were particularly used in dye-sensitized solar cells and in vacuum deposited bilayer solar cells.26,27 PDI molecules possess thermal and photochemical stability, exhibit large optical absorption in the visible to near-infrared spectral region. Some studies28–36 have been devoted to elucidate photo charge generation in polymer/PDIs devices and found that many PDI derivatives do not form good bulk-heterojunction morphologies due to their extended π-surfaces and strong aggregation in the solid state. It indicated that the design of the PDI based molecules should be oriented to create structures with relatively reduced π–π stacking, which contributes to the formation of smaller domains and larger donor–acceptor interface area, allowing excitons to quickly reach a heterojunction. What is more, solid-state packing and/or HOMO and LUMO energy levels of the PDI molecules can be easily specifically tailored either by introduction of appropriate substituents at the imide positions and/or at the perylene core positions.37–39 Under a normal condition, the N-positions of the PDIs are substituted with alkyl chains, preferably branched chains, in order to get high solubility, whereas the core positions are available for some functional groups. And in recent years, people have developed a number of small molecule non-fullerene electron acceptors based on PDI.13–16,33,38,40–49
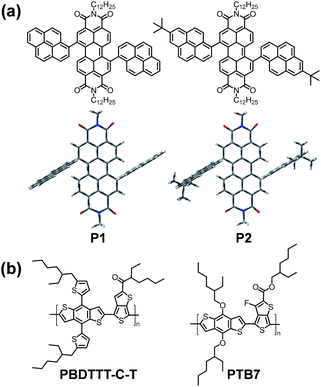
However, the relatively low performance of small molecules may be attributed to their limited interconnectivity through the active layer, resulting in inefficient charge extraction and low short circuit current. Earlier studies have shown that polymers with higher molecular weight (Mn) perform better in BHJ solar cells than lower Mn polymers. Based on the above considerations, the introduction of π-stacking moieties onto the ends of small molecules would facilitate favourable end-to-end π–π interactions, leading to enhanced charge transport between the adjacent molecules. Long-range noncovalent π–π intermolecular interactions may have a strong impact on the molecular self-organization of the PDI molecules in the solid state to enhance the electronic communication between the adjacent chromophores, which may mediate electron and/or energy transfer and thus strongly influence the photovoltaic performance in solar cells. Nevertheless, the π–π intermolecular interaction has to be reduced in order to get suitable PDI-based materials with low aggregation properties for photovoltaic application. Pyrene is a completely planar moiety and has a strong propensity to π-stack. From the consideration on the molecular design, fused aromatic rings like pyrene in bay position may lead to a twisted PDI plane and create a strong steric hindrance, which can decrease the π–π stacking and leads to an almost isolated π system in the solid state. Herein, the effect of the perylene core substitution with fused aromatic rings on the photophysical and photovoltaic parameters is described. We have chosen two electron-rich fused aromatic rings pyrene and 7-tert-butyl-pyrene, as bay substituent groups to develop two new PDI molecules of 1,7-bis(pyrenyl)-N,N′-bis-(n-dodecyl)-perylene-3,4,9,10-bis(dicarboximide) (P1) and 1,7-bis(7-tert-butyl-pyrenyl)-N,N′-bis-(n-dodecyl)-perylene-3,4,9,10-bis(dicarboximide) (P2) (Scheme 1a). The steric hindrance of the pyrene groups induces a distortion of the perylene core and impedes intermolecular π–π stacking of the neighbouring PDI planes. However, the interconnectivity of small molecule semiconductors can be greatly improved by the pyrene end groups. Theoretical calculations indicate that the PDI core with pyrene substituents in the bay positions show low energy molecular conformations characterised by out of plane fused aromatic rings with a dihedral angle of 61° for both P1 and P2, preventing a close contact of the neighbouring PDI planes.
Both materials show good solubility in common organic solvents, such as chloroform, dichloromethane, tetrahydrofuran (THF), and toluene, and can be readily solution-processed to form smooth and pinhole-free films upon spin-coating. They have been used as acceptor components in bulk heterojunction solar cells with PBDTTT-C-T and PTB7 as the donors (Scheme 1b). A PCE of 1.35% was achieved with an open circuit voltage (Voc) of 0.89 V, a short circuit current density (Jsc) of 3.87 mA cm−2 and a fill factor (FF) of 0.39 for the device based on PTB7 and P1. In contrast, the device based on PTB7 and P2 displays a much lower PCE of 0.41%, and it is even lower than that of the device with PBDTTT-C-T as the donor (0.45%) due to the steric hindrance from the bulky tert-butyl.
Experimental
General
1H and 13C NMR spectra were recorded on a Bruker AV-500 spectrometer operating at 500 and 125 MHz, respectively, in deuterated chloroform solution at room temperature. MALDI-TOF spectra were recorded on a Bruker BIFLEX III instrument. Differential scanning calorimetry (DSC) measurements were performed on a Netzsch DSC 209 under a N2 flow at a heating and cooling rate of 10 °C min−1. Thermogravimetric analyses (TGA) were performed on a Netzsch TG 209 under a N2 flow at a heating rate of 10 °C min−1. UV-vis absorption spectra were recorded on a HP 8453 spectrophotometer. Photoluminescence (PL) spectra were measured using a Jobin-Yvon spectrofluorometer. Cyclic voltammetry (CV) was performed on a CHI600D electrochemical workstation with a platinum working electrode and a Pt wire counter electrode at a scanning rate of 100 mV s−1 against a Ag/Ag+ (0.1 M of AgNO3 in acetonitrile) reference electrode with an argon-saturated anhydrous acetonitrile solution of 0.1 M tetrabutylammonium hexafluorophosphate. Thin solid films used for UV-vis absorption and PL spectral measurements were prepared by spin-coating their chlorobenzene solutions on quartz substrates. Atom force microscopy (AFM) measurements were carried out by using a Digital Instrumental DI Multimode Nanoscope IIIa in tapping mode. The processing conditions to make the blend films for morphology study are the same as those for the fabrication of the solar cell devices.Materials
All reactions and manipulations were carried out under inert atmosphere with the use of standard Schlenk techniques. THF was distilled from sodium before use. The other reagents and solvents, unless otherwise specified, were purchased from commercial suppliers and used without further purification. As outlined in Scheme 2, two new PDI molecules P1 and P2 were synthesized through Suzuki coupling reaction.Device fabrication and characterization
Photovoltaic devices were fabricated with a structure of ITO/PFN (10 nm)/photoactive layer (100 nm)/MoO3 (10 nm)/Al (100 nm). Indium tin oxide (ITO)-coated glass substrates were cleaned by sonication in detergent, deionized water, acetone and isopropyl alcohol, and dried in a nitrogen stream, followed by an oxygen plasma treatment. A 10 nm thin PFN layer was spin-coated from its solution in methanol. Subsequently, a ∼100 nm-thin active layer was spin-casted from different donors (PBDTTT-C-T or PTB7) and acceptors (P1 or P2) in a chlorobenzene solution (3% DIO, 20 mg mL−1). Spin-coating was conducted in a nitrogen-filled glove box. Finally, a 10 nm-thin MoO3 layer and a 100 nm-thin Al layer were evaporated through a shadow mask to form a top anode and to define the active area of the devices (0.16 cm2). The active layer thickness was measured using a Dektak 150 profilometer. PCEs were measured in an AM 1.5G solar simulator (Oriel model 91192) under ambient conditions. The power of the sun simulator was calibrated before the testing using a standard silicon solar cell, giving a value of 100 mW cm−1 in the test. The current density–voltage (J–V) characteristics were recorded with a Keithley 2400 source meter. The spectral response was measured with a commercial photomodulation spectroscopic setup (Oriel).Results and discussion
Thermal stability and optical absorption
It is known that the PDI derivatives with symmetrical and unsymmetrical alkyl side chains at the N-terminal position and no substituents on the aromatic core possess excellent thermal stability with high decomposition temperatures.51,52 The thermal property of the developed PDI materials was investigated by TGA and DSC. The TGA traces reported in Fig. 1 show that P1 and P2 show good thermal stability with decomposition temperatures (Td) of 388.7 and 405.1 °C, respectively, as indicated by the temperature corresponding to initial 5% of weight loss in an N2 atmosphere, indicating that they can effectively resist thermal degradation at the operating temperatures in the resultant solar cells. DSC analysis shows that P1 and P2 exhibit glass transition temperatures (Tg) as high as 92.7 and 117.0 °C, respectively, in an N2 atmosphere. P2 has a higher decomposition temperature and glass transition temperature due to its bulky tert-butyl.To study the relationships between the chemical structure and the photophysical property, UV-vis absorption spectra of P1 and P2 in diluted chloroform solutions (10−5 M) and in thin solid films (120 nm) prepared by spin-coating, along with the molar extinction, were recorded as shown in Fig. 2. For both P1 and P2, there is only a small shift in absorption bands, indicating very weak intermolecular interaction in the film state. The spectroscopic feature of P1 and P2 can be associated to the twisted structure of the perylene core induced by the steric hindrance of the fused aromatic pyrene substituents. P1 and P2 in chloroform solution exhibit absorption in the visible range with peak maxima at 495 and 570 nm. The absorption spectra change very slightly in the solid state compared with those in solution. This is an unusual behaviour for the perylene diimide systems, since it is well known that the π–π intermolecular interactions have a strong influence on the optical properties of PDI. Fig. 2b shows individual absorption spectra of the donor and acceptor components in spin-coated films. As shown in the absorption spectra, this donor–acceptor pair has well-matched complementary absorption peaks that completely cover the broad wavelength range from 400 to 750 nm.
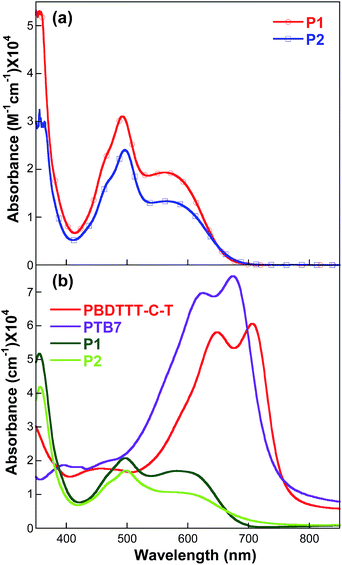 | ||
| Fig. 2 UV-vis absorption spectra of (a) P1 and P2 in chloroform solutions; (b) P1, P2, PBDTTT-C-T and PTB7 in thin solid films. | ||
Electrochemical properties and energy levels
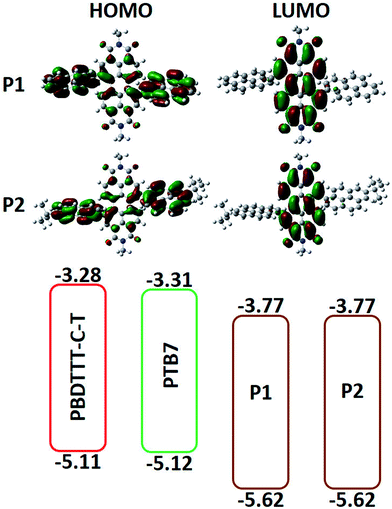
In order to insightfully understand the relationships between the chemical structure and the electronic structure of the resulting materials and consequently provide key parameters for the design of small molecule non-fullerene BHJ solar cells, CV experiments were conducted to measure HOMO (highest occupied molecular orbitals) and LUMO (lowest unoccupied molecular orbitals) energy levels of P1 and P2. The potentials were calibrated with the redox couple of ferrocene/ferrocenium (Fc/Fc+) under the same experimental conditions. In the oxidation and reduction curves shown in Fig. 3, both the CV curves of P1 and P2 in acetonitrile solution show one irreversible p-doping and n-doping process. The onset oxidation potentials (Eox) versus Ag/AgNO3 are 1.22 V for both P1 and P2, and the onset reduction potentials (Ered) versus Ag/AgNO3 are −0.63 V for both P1 and P2. As summarized in Table 1, HOMO and LUMO energy levels of P1 and P2 are estimated to be −5.62 and −3.77 eV, respectively, according to an equation of EHOMO = −e(Eox + 4.54) (eV).53 Considering the same conjugated main chain structure, their frontier energy levels are almost the same or change little when different end-groups are introduced. The relatively low HOMO and LUMO energy levels of the current PDI compounds can be ascribed to the PDI unit having stronger electron affinity. | ||
| Fig. 3 CV curves of the P1 and P2 films measured in an anhydrous acetonitrile solution of 0.1 M Bu4NPF6 with a scan rate of 100 mV s−1. | ||
Theoretical calculations
In order to study the ground-state geometries and electronic structures of P1 and P2, density functional theory (DFT) calculations have also been performed by using Gaussian 03W program based on B3LYP/6-31G(d, p) basis set. To expedite the calculation, the alkyl chains on the PDI nitrogen were exchanged with methyl groups to give model compounds. Fig. 4a shows the electron distributions of the HOMOs and LUMOs of the ground-state optimized structures for P1 and P2 systems. Since they have the same conjugated main chain structure, the frontier energy levels of P1 and P2 are almost the same when different terminal groups are introduced. As indicated in Fig. 4a, the electron densities of the HOMOs of P1 and P2 are delocalized on the whole molecule. On the other hand, the electron densities of the LUMOs of P1 and P2 are mainly localized on the PDI moieties due to its strong electron affinity. The calculated HOMO and LUMO energy levels of the ground-state optimized geometries of P1 and P2 are −5.43/−5.37 eV and −3.28/−3.24 eV, respectively. The theoretically estimated values refer to the gas phase with isolated molecules, whereas the experimental values are for the condensed phase, and so the values are somewhat different.Photovoltaic properties
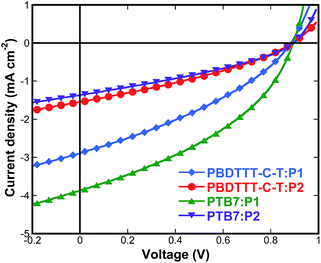
To demonstrate the potential of these two small molecules as an electron acceptor material in organic solar cells, photovoltaic devices with a structure of ITO/PFN (10 nm)/photoactive layer (100 nm)/MoO3 (10 nm)/Al (100 nm) were fabricated by spin-coating from their chlorobenzene solutions at a concentration of 20 mg mL−1 in a mixture with PBDTTT-C-T and PTB7 (3% DIO) as the donor materials. Typical current density–voltage (J–V) characteristics of the fabricated devices under 1 sun illumination (AM 1.5G, 100 mW cm−2) are displayed in Fig. 5, and the device performances are summarized in Table 2.| Active layer | Voc (V) | Jsc (mA cm−2) | FF (%) | PCE (%) |
|---|---|---|---|---|
PBDTTT-C-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P1 P1 |
0.88 | 2.89 | 34.4 | 0.88 |
PBDTTT-C-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2 P2 |
0.89 | 1.54 | 32.6 | 0.45 |
PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P1 P1 |
0.89 | 3.87 | 39.3 | 1.35 |
PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2 P2 |
0.90 | 1.36 | 33.0 | 0.41 |
Considering the HOMO and LUMO energy levels of PBDTTT-C-T or PTB7 and the current acceptors (Fig. 4b), there is sufficient driving force to form charge carriers. From Fig. 5 and Table 2, one can see the characteristically high Voc of the devices based on P1 and P2 (>0.8 V) because of their relatively higher-lying LUMO energy levels, which is predominantly determined by the backbone acceptor moiety. For the devices with PBDTTT-C-T as the donor material, the active layer of PBDTTT-C-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P1 with a weight ratio of 1
P1 with a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 exhibited a high Voc of 0.88 V, a Jsc of 2.89 mA cm−2, a FF of 0.34, and a PCE of 0.88%. In contrast, the active layer based on PBDTTT-C-T
1 exhibited a high Voc of 0.88 V, a Jsc of 2.89 mA cm−2, a FF of 0.34, and a PCE of 0.88%. In contrast, the active layer based on PBDTTT-C-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2 with the same weight ratio gave a Voc of 0.89 V, a Jsc of 1.54 mA cm−2, a FF of 0.33, and a PCE of 0.45%. By using PTB7 instead of PBDTTT-C-T as the donor material, the photovoltaic devices based on PTB7
P2 with the same weight ratio gave a Voc of 0.89 V, a Jsc of 1.54 mA cm−2, a FF of 0.33, and a PCE of 0.45%. By using PTB7 instead of PBDTTT-C-T as the donor material, the photovoltaic devices based on PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P1 (1
P1 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) with a thickness of 100 nm showed a PCE value up to 1.35% with a Voc of 0.89 V, a significantly improved Jsc of 3.87 mA cm−2, and a FF of 0.39. In comparison, the devices based on PTB7
1, w/w) with a thickness of 100 nm showed a PCE value up to 1.35% with a Voc of 0.89 V, a significantly improved Jsc of 3.87 mA cm−2, and a FF of 0.39. In comparison, the devices based on PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2 (1
P2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) exhibited a slightly higher Voc of 0.90 V, a very low Jsc of 1.36 mA cm−2, a FF of 0.33, and a PCE of only 0.41%, which is even lower than the devices with PBDTTT-C-T as the donor. Compared to the OSCs based on PBDTTT-C-T
1, w/w) exhibited a slightly higher Voc of 0.90 V, a very low Jsc of 1.36 mA cm−2, a FF of 0.33, and a PCE of only 0.41%, which is even lower than the devices with PBDTTT-C-T as the donor. Compared to the OSCs based on PBDTTT-C-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2 and PTB7
P2 and PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2, the higher PCE of the PBDTTT-C-T
P2, the higher PCE of the PBDTTT-C-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P1 and PTB7
P1 and PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P1-based cells can be primarily explained by their higher Jsc. Jsc is known to depend largely on exciton diffusion, charge separation/recombination, and balanced transport of holes and electrons through the device active layer. Pyrene is a completely planar moiety and has a strong propensity to π-stack. P1 with pyrene terminal groups make for the interconnectivity of small molecule semiconductors, thus give a favourable donor–acceptor interpenetrating network (IPN). The well-defined IPN structure ensures large D–A interfaces and efficient percolation channels for charge transport, thus improving the exciton separation and carrier collection efficiency and leading to a high Jsc and FF. However, P2 with tert-butyl-pyrene terminal groups disfavour π–π interactions between the neighbouring molecules and led to a lower Jsc. These results suggest that, relative to the tert-butyl-pyrene terminal groups, the pyrene end-group affects intermolecular interactions which may promote molecular packing and active layer morphology favourable for high device PCE.
P1-based cells can be primarily explained by their higher Jsc. Jsc is known to depend largely on exciton diffusion, charge separation/recombination, and balanced transport of holes and electrons through the device active layer. Pyrene is a completely planar moiety and has a strong propensity to π-stack. P1 with pyrene terminal groups make for the interconnectivity of small molecule semiconductors, thus give a favourable donor–acceptor interpenetrating network (IPN). The well-defined IPN structure ensures large D–A interfaces and efficient percolation channels for charge transport, thus improving the exciton separation and carrier collection efficiency and leading to a high Jsc and FF. However, P2 with tert-butyl-pyrene terminal groups disfavour π–π interactions between the neighbouring molecules and led to a lower Jsc. These results suggest that, relative to the tert-butyl-pyrene terminal groups, the pyrene end-group affects intermolecular interactions which may promote molecular packing and active layer morphology favourable for high device PCE.
To further understand the device performance, the EQE spectra of the fabricated devices were measured as shown in Fig. 6. From the curves, it is observed that all the EQE spectra cover a broad wavelength range from 300 to 800 nm. The calculated Jsc values obtained by the integration of the EQE data for the PBDTTT-C-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P1 and PTB7
P1 and PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P1 devices showed a 3–6% mismatch compared with the Jsc values obtained from the J–V measurements. Obviously, the EQE of the devices based on PTB7
P1 devices showed a 3–6% mismatch compared with the Jsc values obtained from the J–V measurements. Obviously, the EQE of the devices based on PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P1 is higher than that of the devices based on the blend of PBDTTT-C-T
P1 is higher than that of the devices based on the blend of PBDTTT-C-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P1, and it can be attributed to the stronger light absorption of PTB7 and the appropriate film morphology as proven by the following AFM images.
P1, and it can be attributed to the stronger light absorption of PTB7 and the appropriate film morphology as proven by the following AFM images.
Film morphology
In order to deeply understand the photovoltaic properties of the resulting small molecular materials, the active layer morphology was studied by AFM in the tapping-mode and transmission electron microscopy (TEM). As shown by the images presented in Fig. 7 and 8, it can be obviously seen that the different terminal groups of these two small molecules result in substantial morphology variation in the condensed state. The root-mean-square (rms) roughness of the PBDTTT-C-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P1, PBDTTT-C-T
P1, PBDTTT-C-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2, PTB7
P2, PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P1, PTB7
P1, PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2 films are 1.566, 1.618, 0.964 and 1.132 nm, respectively. The surfaces of the P1-based films are quite smooth and uniform. The phase segregation for the blended films of P2 is negligible, while the phase segregation and aggregation for the blended films of P1 are more obvious, indicating that P1 may have a good miscibility with both PBDTTT-C-T and PTB7 to give a favourable donor–acceptor interpenetrating network (IPN). The well-defined IPN structure ensures large D–A interfaces and efficient percolation channels for charge transport, thus improving the exciton separation and carrier collection efficiency and leading to a high Jsc and FF. In comparison, the films blended with P2 exhibit a larger roughness and less phase separation, which indicates an unfavourable film morphology that is a major disadvantage for charge separation from the donor to the acceptor, thus leading to lower Jsc and FF. From a structural point of view, there might be π–π intermolecular interactions between the pyrene groups of the P1 molecules and PBDTTT-C-T or PTB7 that may improve their miscibility with each other, leading to desirable film morphology for exciton separation and charge transport.
P2 films are 1.566, 1.618, 0.964 and 1.132 nm, respectively. The surfaces of the P1-based films are quite smooth and uniform. The phase segregation for the blended films of P2 is negligible, while the phase segregation and aggregation for the blended films of P1 are more obvious, indicating that P1 may have a good miscibility with both PBDTTT-C-T and PTB7 to give a favourable donor–acceptor interpenetrating network (IPN). The well-defined IPN structure ensures large D–A interfaces and efficient percolation channels for charge transport, thus improving the exciton separation and carrier collection efficiency and leading to a high Jsc and FF. In comparison, the films blended with P2 exhibit a larger roughness and less phase separation, which indicates an unfavourable film morphology that is a major disadvantage for charge separation from the donor to the acceptor, thus leading to lower Jsc and FF. From a structural point of view, there might be π–π intermolecular interactions between the pyrene groups of the P1 molecules and PBDTTT-C-T or PTB7 that may improve their miscibility with each other, leading to desirable film morphology for exciton separation and charge transport.
 | ||
Fig. 7 Tapping mode AFM topography images (5 × 5 μm2) of (a) PBDTTT-C-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P1, (b) PBDTTT-C-T P1, (b) PBDTTT-C-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2, (c) PTB7 P2, (c) PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P1, and (d) PTB7 P1, and (d) PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2. P2. | ||
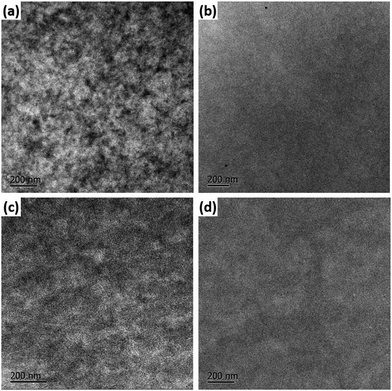 | ||
Fig. 8 TEM images of (a) PBDTTT-C-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P1, (b) PBDTTT-C-T P1, (b) PBDTTT-C-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2, (c) PTB7 P2, (c) PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P1, and (d) PTB7 P1, and (d) PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2 BHJ blend films. P2 BHJ blend films. | ||
Photoluminescence characterization
In order to obtain insight into the charge transfer process in the donor/acceptor blends, PL spectra of the neat films of P1, P2, PBDTTT-C-T, PTB7 and the blend films of PBDTTT-C-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P1, PBDTTT-C-T
P1, PBDTTT-C-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2, PTB7
P2, PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P1, and PTB7
P1, and PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2 were investigated. The emission spectra were obtained at excitation wavelength of 640, 620, 490, and 490 nm for the films based on PBDTTT-C-T, PTB7, P1, and P2, respectively, which are the strongest absorption wavelength for each film. Fig. 9b reveals that there is only a small shift for the PL spectra measured in solution and film state for both P1 and P2, suggesting the absence of any strong π–π interactions. Fig. 9c and d reveals that the PL intensity of the PBDTTT-C-T and PTB7 films is dramatically quenched by the addition of P1 and P2. Similarly, the PL intensity of the P1 and P2 films is also dramatically quenched by the addition of PBDTTT-C-T and PTB7. It is shown that the PL intensity of the blend films changes little when different end groups are introduced. In contrast, the quenching effect for the blend films of PTB7
P2 were investigated. The emission spectra were obtained at excitation wavelength of 640, 620, 490, and 490 nm for the films based on PBDTTT-C-T, PTB7, P1, and P2, respectively, which are the strongest absorption wavelength for each film. Fig. 9b reveals that there is only a small shift for the PL spectra measured in solution and film state for both P1 and P2, suggesting the absence of any strong π–π interactions. Fig. 9c and d reveals that the PL intensity of the PBDTTT-C-T and PTB7 films is dramatically quenched by the addition of P1 and P2. Similarly, the PL intensity of the P1 and P2 films is also dramatically quenched by the addition of PBDTTT-C-T and PTB7. It is shown that the PL intensity of the blend films changes little when different end groups are introduced. In contrast, the quenching effect for the blend films of PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P1 and PTB7
P1 and PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2 is more efficient than the corresponding blend films of PBDTTT-C-T
P2 is more efficient than the corresponding blend films of PBDTTT-C-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P1 and PBDTTT-C-T
P1 and PBDTTT-C-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2 with the same weight ratio. Analogously, the quenching effect for the P1
P2 with the same weight ratio. Analogously, the quenching effect for the P1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PBDTTT-C-T or PBT7 blend films is more efficient than the blend films of P2
PBDTTT-C-T or PBT7 blend films is more efficient than the blend films of P2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PBDTTT-C-T or PTB7. The results indicate that photo-induced charge transfer from PTB7 to P1 should be more efficient to be as an electron acceptor in organic photovoltaic devices.
PBDTTT-C-T or PTB7. The results indicate that photo-induced charge transfer from PTB7 to P1 should be more efficient to be as an electron acceptor in organic photovoltaic devices.
Conclusions
In summary, we designed, synthesized and characterized two novel perylene diimide molecules, P1 and P2, with fused aromatic pyrene rings on the perylene core. These materials possess interesting optical and structural features, revealing extremely weak π–π interactions between the perylene planes, which is an interesting feature, since PDIs are well known for their strong intermolecular packing properties. DFT calculations show that the bay-substitutions introduce a twisting of the perylene core. Aside from the good solubility and stability, effective PL quenching was observed when PBDTTT-C-T or PTB7 is blended with the developed PDI molecules, indicating efficient charge/energy transfer occurred and the two molecular materials could be used as acceptors in solution-processable organic solar cells. Compared with P2, P1 gives a favourable blend film morphology with PTB7 as the donor material for efficient exciton separation and charge transport, leading to a relatively high PCE of 1.35% under the illumination of AM 1.5G, 100 mW cm−2.Acknowledgements
The authors greatly appreciate the financial support from the Ministry of Science and Technology (2014CB643501, 2015CB655003 and 2014DFA52030), the National Natural Science Foundation of China (91233116 and 51073057), the Ministry of Education (NCET-11-0159), and the Guangdong Natural Science Foundation (S2012030006232).References
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789 CAS
.
- H. Y. Chen, J. H. Hou, S. Q. Zhang, Y. Y. Liang, G. W. Yang, Y. Yang, L. P. Yu, Y. Wu and G. Li, Nat. Photonics, 2009, 3, 649 CrossRef CAS PubMed
.
- X. G. Guo, N. J. Zhou, S. J. Lou, J. Smith, D. B. Tice, J. W. Hennek, R. P. Ortiz, J. T. L. Navarrete, S. Y. Li, J. Strzalka, L. X. Chen, R. P. H. Chang, A. Facchetti and T. J. Marks, Nat. Photonics, 2013, 7, 825 CrossRef CAS PubMed
.
- Z. C. He, C. M. Zhong, S. J. Su, M. Xu, H. B. Wu and Y. Cao, Nat. Photonics, 2012, 6, 591 Search PubMed
.
- J. B. You, L. T. Dou, K. Yoshimura, T. Kato, K. Ohya, T. Moriarty, K. Emery, C. C. Chen, J. Gao, G. Li and Y. Yang, Nat. Commun., 2013, 4, 1446 CrossRef PubMed
.
- B. Parida, S. Iniyan and R. Goic, Renewable Sustainable Energy Rev., 2011, 15, 1625 CrossRef CAS PubMed
.
- J. D. Myers and J. Xue, Polym. Rev., 2012, 52, 1 CrossRef CAS PubMed
.
- S. H. Liao, H. J. Jhuo, Y. S. Cheng and S. A. Chen, Adv. Mater., 2013, 25, 4766 CrossRef CAS PubMed
.
- L. Ye, W. Zhang, H. Zhao and J. Hou, Chem. Mater., 2014, 26, 3603 CrossRef CAS
.
- C.-Z. Li, C.-Y. Chang, Y. Zang, H.-X. Ju, C.-C. Chueh, P.-W. Liang, N. Cho, D. S. Ginger and A. K. Y. Jen, Adv. Mater., 2014, 26, 6262 CrossRef CAS PubMed
.
- Y. He and Y. Li, Phys. Chem. Chem. Phys., 2011, 13, 1970 RSC
.
- A. Anctil, C. W. Babbitt, R. P. Raffaelle and B. J. Landi, Environ. Sci. Technol., 2011, 45, 2353 CrossRef CAS PubMed
.
- X. Zhang, Z. Lu, L. Ye, C. Zhan, J. Hou, S. Zhang, B. Jiang, Y. Zhao, J. Huang, S. Zhang, Y. Liu, Q. Shi, Y. Liu and J. Yao, Adv. Mater., 2013, 25, 5791 CrossRef CAS PubMed
.
- W. Jiang, L. Ye, X. G. Li, C. Y. Xiao, F. Tan, W. C. Zhao, J. H. Hou and Z. H. Wang, Chem. Commun., 2014, 50, 1024 RSC
.
- Z. H. Lu, B. Jiang, X. Zhang, A. L. Tang, L. L. Chen, C. L. Zhan and J. N. Yao, Chem. Mater., 2014, 26, 2907 CrossRef CAS
.
- Y. Lin, Y. Wang, J. Wang, J. Hou, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2014, 26, 5137 CrossRef CAS PubMed
.
- J. T. Bloking, T. Giovenzana, A. T. Higgs, A. J. Ponec, E. T. Hoke, K. Vandewal, S. Ko, Z. Bao, A. Sellinger and M. D. McGehee, Adv. Energy Mater., 2014, 4, 1301426 Search PubMed
.
- L. Ye, W. Jiang, W. Zhao, S. Zhang, D. Qian, Z. Wang and J. Hou, Small, 2014, 22, 4658 CrossRef PubMed
.
- Z. Mao, W. Senevirathna, J.-Y. Liao, J. Gu, S. V. Kesava, C. Guo, E. D. Gomez and G. Sauvé, Adv. Mater., 2014, 26, 6290 CrossRef CAS PubMed
.
- Y. Zang, C. Z. Li, C. C. Chueh, S. T. Williams, W. Jiang, Z. H. Wang, J. S. Yu and A. K. Jen, Adv. Mater., 2014, 26, 5708 CrossRef CAS PubMed
.
- J. Zhao, Y. Li, H. Lin, Y. Liu, K. Jiang, C. Mu, T. Ma, J. Y. L. Lai and H. Yan, Energy Environ. Sci., 2015, 8, 520 CAS
.
- X. Zhang, C. L. Zhan and J. Yao, Chem. Mater., 2015, 27, 166 CrossRef CAS
.
- Y. Zhong, M. T. Trinh, R. S. Chen, W. Wang, P. Khlyabich, B. Kumar, Q. Z. Xu, C.-Y. Nam, M. Sfeir, C. Black, M. Steigerwald, Y. L. Loo, S. X. Xiao, F. Ng, X.-Y. Zhu and C. Nuckolls, J. Am. Chem. Soc., 2014, 136, 15215 CrossRef CAS PubMed
.
- Y. Z. Lin, Z.-G. Zhang, H. T. Bai, J. Y. Wang, Y. H. Yao, Y. F. Li, D. B. Zhu and X. W. Zhan, Energy Environ. Sci., 2015, 8, 610 CAS
.
- Y. Z. Lin, J. Y. Wang, Z.-G. Zhang, H. T. Bai, Y. F. Li, D. B. Zhu and X. W. Zhan, Adv. Mater., 2015, 27, 1170 CrossRef CAS PubMed
.
- S. Ferrere and B. A. Gregg, New J. Chem., 2002, 26, 1155 RSC
.
- R. Tettignies, Y. Nicolas, P. Blanchard, E. Levillain, J.-M. Nunzi and J. Roncali, Adv. Mater., 2003, 15, 1939 CrossRef PubMed
.
- P. E. Keivanidis, I. A. Howard and R. H. Friend, Adv. Funct. Mater., 2008, 18, 3189 CrossRef CAS PubMed
.
- S. Shoaee, Z. An, X. Zhang, S. Barlow, S. R. Marder, W. Duffy, M. Heeney, I. McCulloch and J. R. Durrant, Chem. Commun., 2009, 5445 RSC
.
- I. A. Howard, F. Laquai, P. E. Keivanidis, R. H. Friend and N. C. Greenham, J. Phys. Chem. C, 2009, 113, 21225 CAS
.
- Z. Chen, A. Lohr, C. R. Saha-Möller and F. Würthner, Chem. Soc. Rev., 2009, 38, 564 RSC
.
- S. Shoaee, T. M. Clarke, C. Huang, S. Barlow, S. R. Marder, M. Heeney, I. McCulloch and J. R. Durrant, J. Am. Chem. Soc., 2010, 132, 12919 CrossRef CAS PubMed
.
- V. Kamm, G. Battagliarin, I. A. Howard, W. Pisula, A. Mavrinskiy, C. Li, K. Müllen and F. Laquai, Adv. Energy Mater., 2011, 1, 297 CrossRef CAS PubMed
.
- E. Kozma, D. Kotowski, M. Catellani, S. Luzzati, A. Famulari and F. Bertini, Dyes Pigm., 2013, 99, 329 CrossRef CAS PubMed
.
- M. G. Li, L. Wang, J. G. Liu, K. Zhou, X. H. Yu, R. B. Xing, Y. H. Geng and Y. C. Han, Phys. Chem. Chem. Phys., 2014, 16, 4528 RSC
.
- P. E. Hartnett, A. Timalsina, H. S. S. Ramakrishna Matte, N. J. Zhou, X. G. Guo, W. Zhao, A. Facchetti, R. P. H. Chang, M. C. Hersam, M. R. Wasielewski and T. J. Marks, J. Am. Chem. Soc., 2014, 136, 16345 CrossRef CAS PubMed
.
- J. L. Segura, H. Herrera and P. Bauerle, J. Mater. Chem., 2012, 22, 8717 RSC
.
- F. Wurtner, V. Stepanenko, Z. Chen, C. R. Saha-Moller, N. Kocher and D. Stalke, J. Org. Chem., 2004, 69, 7933 CrossRef PubMed
.
- Z. Chen, U. Baumeister, C. Tschierske and F. Wurtner, Chem.–Eur. J., 2007, 13, 450 CrossRef CAS PubMed
.
- Z. Tan, E. Zhou, X. Zhan, X. Wang, Y. Li, S. Barlow and S. R. Marder, Appl. Phys. Lett., 2008, 93, 073309 CrossRef PubMed
.
- Y. Liu, T. T. Larsen-Olsen, X. Zhao, B. Andreasen, R. R. Søndergaard, M. Helgesen, K. Norrman, M. Jørgensen, F. C. Krebs and X. Zhan, Sol. Energy Mater. Sol. Cells, 2013, 112, 157 CrossRef CAS PubMed
.
- Y. Zhou, T. Kurosawa, W. Ma, Y. Guo, L. Fang, K. Vandewal, Y. Diao, C. Wang, Q. Yan, J. Reinspach, J. Mei, A. L. Appleton, G. I. Koleilat, Y. Gao, S. C. B. Mannsfeld, A. Salleo, H. Ade, D. Zhao and Z. Bao, Adv. Mater., 2014, 26, 3767 CrossRef CAS PubMed
.
- E. J. Zhou, J. Z. Cong, Q. S. Wei, K. Tajima, C. H. Yang and K. Hashimoto, Angew. Chem., Int. Ed., 2011, 50, 2799 CrossRef CAS PubMed
.
- F. Nolde, W. Pisula, S. Müller, C. Kohl and K. Müllen, Chem. Mater., 2006, 18, 3715 CrossRef CAS
.
- J. Pommerehne, H. Vestweber, W. Guss, R. F. Mahrt, H. Bassler, M. Porsch and J. Daub, Adv. Mater., 1995, 7, 551 CrossRef CAS PubMed
.
- A. Sharenko, C. M. Proctor, T. S. van der Poll, Z. B. Henson, T.-Q. Nguyen and G. C. Bazan, Adv. Mater., 2013, 25, 4403 CrossRef CAS PubMed
.
- R. Shivanna, S. Shoaee, S. Dimitrov, S. K. Kandappa, S. Rajaram, J. R. Durrant and K. S. Narayan, Energy Environ. Sci., 2014, 7, 435 CAS
.
- Q. Yan, Y. Zhou, Y.-Q. Zheng, J. Pei and D. Zhao, Chem. Sci., 2013, 4, 4389 RSC
.
- X. Liu, P. Cai, D. C. Chen, J. W. Chen, S. J. Su and Y. Cao, Sci. China: Chem., 2014, 57, 973 CrossRef CAS
.
- X. Liu, S. J. Su and Y. Cao, Polym. Bull., 2014, 12, 68 Search PubMed
.
- X. Zhan, Z. Tan, B. Domercq, Z. An, X. Zhang, S. Barlow, Y. Li, D. Zhu, B. Kippelen and S. R. Marder, J. Am. Chem. Soc., 2007, 129, 7246 CrossRef CAS PubMed
.
- X. Zhan, A. Facchetti, S. Barlow, T. J. Marks, M. A. Ratner, M. R. Wasielewski and S. R. Marder, Adv. Mater., 2011, 23, 268 CrossRef CAS PubMed
.
- P. Cheng, L. Ye, X. Zhao, J. Hou, Y. Li and X. Zhan, Energy Environ. Sci., 2014, 7, 1351 CAS
.
| This journal is © The Royal Society of Chemistry 2015 |