Different acidity and additive effects of zirconium metal–organic frameworks as catalysts for cyanosilylation
Fu-Gui Xi,
Yang Yang,
Hui Liu,
Hong-Fei Yao and
En-Qing Gao*
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, College of Chemistry and Molecular Engineering, East China Normal University, Shanghai 200062, P. R. China. E-mail: eqgao@chem.ecnu.edu.cn; Fax: +86-21-62233404; Tel: +86-21-62233404
First published on 11th September 2015
Abstract
The Zr(IV) metal–organic framework with 1,4-benzenedicarboxylate (UiO-66) in different forms was studied as a solid catalyst for carbonyl cyanosilylation. The anhydrous material (UiO-66-A) obtained after calcination has open Lewis-acid sites and acts as a heterogeneous and size selective catalyst for the reaction of aldehydes and trimethylsilylcyanide (TMSCN). Notably, it was found that the as-synthesized hydrous form (UiO-66-H) shows comparable activity to UiO-66-A, so UiO-66 can be used as a catalyst for cyanosilylation with no need of high-temperature activation. With a number of intentionally designed control experiments, we demonstrated that the acetic acid enclosed in UiO-66-H during synthesis serves as a Brønsted acid to promote the reaction, though acetic acid is inactive by itself. The different acidity between UiO-66-H and UiO-66-A was confirmed by using the isomerization of α-pinene oxide as a probe reaction. Both UiO-66-H and UiO-66-A are recyclable without significant degradation in framework integrity and catalytic activity. In addition, it was unexpectedly found that pyridine, which is inactive alone, acts as co-catalyst, rather than a Lewis acid poison, to dramatically accelerate the catalytic reaction over UiO-66-H or UiO-66-A. A synergistic mechanism was suggested, in which the Lewis or Brønsted acid activates the aldehyde substrate while pyridine acts as a Lewis base to activate TMSCN.
Introduction
Metal–organic frameworks (MOFs) have emerged as a new type of porous materials in recent years. By judicious choice and design of the metal and organic components and/or by targeted post-synthetic modifications, not only can the frameworks have high surface area and porosity, but they can also be very rich in structure and functionality.1,2 So MOFs have a broad application prospect in gas adsorption/separation,3,4 drug delivery,5 and sensing applications.6 Especially the applications of MOFs in heterogeneous catalysis have attracted much attention, and it is believed that MOFs can serve as alternatives or complement conventional porous materials such as zeolites in many catalytic processes.7–11 The catalytic active sites in MOFs, either inherent in the frameworks or introduced by post-synthetic modifications,10,12,13 can be metal centres or various functional groups attached to the inorganic or organic components of the frameworks.10,12 Besides, open cavities/channels of MOFs can serve as catalyst carriers and/or confined reactors.14–18The catalytic applications of MOFs are often limited by their thermal and chemical stabilities. Some MOFs with trivalent metal ions such as Al(III), Cr(III) and Fe(III), namely, the MIL-53, MIL-100, and MIL-101 series, are among the most stable MOFs and have been tested as reusable heterogeneous catalysts or catalyst hosts for various organic reactions.19–30 Some isoreticular Zr(IV) MOFs of general formula [Zr6O4(OH)4(L)6] [L = 1,4-benzenedicarboxylate (BDC, for the prototypic UiO-66) and analogous aromatic dicarboxylates, functionalized or elongated] have also been shown to have exceptional stability.31–34 These 3D MOFs are based on octahedral [Zr6(μ3-O)4(μ3-OH)4(μ2-COO)12] clusters (Fig. 1a) and their porous systems feature alternating octahedral and tetrahedral cages sharing triangular windows (Fig. 1b). Upon calcination, the cluster becomes [Zr6(μ3-O)6(μ2-COO)12] by releasing two water molecules, and the Zr centres change from eight- to seven-coordinated, generating potential Lewis-acid sites. However, catalytic studies with the UiO-66 series are still relatively rare. Vermoortele et al. demonstrated that UiO-66 is active for the cross-aldol condensation between benzaldehyde and heptanal and that the isoreticular NH2-functionalized MOF (UiO-66-NH2) performs better due to cooperative effects.33 The same research group found that the catalytic activity of UiO-66-type MOFs for citronellal cyclization can be increased by functionalizing the BDC linker with electron-withdrawing groups or by using trifluoroacetic acid as synthetic modulator;35,36 Kim et al. demonstrated that UiO-66 and UiO-66-NH2 are more active than MIL-101, CuBTC, ZIF-8, MOF-5 and IRMOF-3 for CO2 cycloaddition to styrene oxide.37 More recently, the two MOFs have been studied as active and stable catalysts for the esterification of levulinic acid with biomass derived alcohols,38 and UiO-66-NH2 has been found to be very active for phosphate-ester hydrolysis owing to the proton-transfer function of the amino moiety.39 The Zr MOFs have also been tested as photocatalysts for hydrogen generation from water/methanol and for oxidation of organic compounds.40–46
Carbonyl cyanosilylation represents a convenient synthetic route to cyanohydrins, which are versatile intermediates in organic synthesis. The reaction can be promoted by various Lewis acids and bases.47–49 In particular, cyanosilylation of aldehydes does not require strong Lewis acidity and can be carried out under mild conditions, affording a suitable model reaction for MOF catalysis.10,11 The first use of a MOF for catalytic cyanosilylation was reported in 1994,50 and there has been a surge in new examples over the last few years, involving diverse MOF structures with Cd(II), Cu(II), Mn(II), Zn(II), Al(III), Cr(III), Ln(III) or Sc(III) as Lewis acid sites.51–60 However, most of the studies have been limited to the demonstration of the catalytic activity.
Here we report a detailed catalytic study with different forms of UiO-66 in presence/absence of additives. The results show that both hydrous and anhydrous forms (denoted as UiO-66-H and UiO-66-A, respectively) of the material are recyclable heterogeneous catalysts for cyanosilylation of aldehydes with trimethylsilylcyanide (TMSCN). We demonstrate that the catalytic activity of UiO-66-H and UiO-66-A originates from different acidity (Brønsted or Lewis). Furthermore, it was unexpectedly found that pyridine, which is inactive alone, acts as co-catalyst, rather than poison, to dramatically promote UiO-66 catalyzed cyanosilylation reactions.
Experimental
Synthesis
All chemicals are obtained from SCRC and used without further purification. The synthesis of UiO-66 was carried out by following a solvothermal procedure reported in the literature,61,62 using acetic acid as modulating reagent. ZrCl4 (0.7 mmol, 0.164 g) and H2BDC (0.7 mmol, 0.116 g) were dissolved in the mixture of N,N′-dimethylformamide (DMF, 8 mL) and acetic acid (1.2 mL) at room temperature. After stirring for about 5 minutes, the mixture was sealed in a 23 mL Teflon liner, heated in an oven at 120 °C for 48 hours, and then cooled to room temperature. A white solid powder was isolated by filtration, washed three times with DMF and dried at 70 °C in an oven. The product thus obtained is hereafter denoted as UiO-66-H (H = hydrous), for which the Zr6 cluster is in the hydrous form [Zr6(μ3-O)4(μ3-OH)4] and solvent molecules (water, DMF and acetic acid) are not evacuated from the pores. To obtain the evacuated and dehydrated material denoted as UiO-66-A (A = anhydrous), UiO-66-H was calcinated at 300 °C for 2 hours.Characterization
The X-ray powder diffraction patterns of the samples were measured using a Rigaku Ultima IV X-ray Diffractometer with Cu Kα radiation (λ = 1.54 Å) at a scanning rate of 10 °C min−1, with accelerating voltage and current of 35 kV and 25 mA, respectively. Thermogravimetric analysis (TGA) was performed on a STA 449 F3 Simultaneous Thermal Analyzer in the temperature range from 50 to 800 °C at a rate of 10 °C min−1 under an air flow. Gas chromatography (GC) was conducted using a LingHua GC 9890E instrument equipped with an FID detector. The 1H NMR spectroscopy was performed on a Bruker DRX500 spectrometer (500 MHz with TMS as reference).Catalytic test
In a typical catalytic experiment, about 0.5 mmol (referring to the Zr content) of UiO-66-A (0.135 g; the molecular weight (MW) is 271 according to the simplest formula ZrO(BDC)) or UiO-66-H (0.205 g; the MW was estimated to be 410 from TGA) was placed in a two-necked round-bottom flask connected to a reflux condenser. After three cycles of vacuum pumping and nitrogen injection, the solution of trimethylsilylcyanide (TMSCN, 10 mmol) in 10 mL DCM was added and heated to reflux. Then a solution of benzaldehyde (5 mmol) and n-dodecane (5 mmol, as internal standard) in 5 mL DCM was added with stirring. The reaction mixture was analyzed by GC at different time intervals.Results and discussion
General characterization of the catalysts
The XRD patterns of UiO-66-H and UiO-66-A are shown in Fig. 2b and c. The patterns are in good agreement with those simulated from the crystallographic data of UiO-66,31,63 indicating the successful preparation of the MOFs and the retention of the structural integrity after dehydration at 300 °C. Note that the weak [220] peak observed at about 12° for UiO-66-H almost disappears in the pattern of UiO-66-A. This is because the peak mainly arises from the scattering of the guest molecules enclosed in the pores. Therefore, the peak may be taken as an indication of the presence of guests in the pores.The TGA curve measured in air flux for the hydrous material is shown in Fig. 3. The large weight loss of about 34% up to 300 °C can be attributed to the evacuation and dehydration of the material. The weight loss is sensitive to post-synthetic treatment procedures (washing, solvent exchange and drying) applied to the sample, and thus the stoichiometry of the guest molecules (water, DMF and acetic acid) in the pores cannot be exactly defined.63 After a relative plateau in the TGA profile, the material undergoes a final rapid weight loss (33%) starting at about 480 °C and ending at 560 °C, which can be attributed to the decomposition of the BDC ligand. The final high-temperature residue (33%) is assumed to be ZrO2. From these data, the Zr content in the anhydrous material can be calculated to be 37%. This value is somewhat higher than the value of 33.6% calculated according to the expected formula [Zr6O6(BDC)6]. The difference may be due to the presence of defects (random missing of the BDC linkers) in the framework.35,36,63,64
Catalytic properties of UiO-66-A
The cyanosilylation of benzaldehyde was selected as a model reaction to confirm the catalytic activity of UiO-66 and to optimize the reaction conditions. Some results are collected in Table 1. The test reaction with an excess of TMSCN (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) in the presence of UiO-66-A yielded 2-phenyl-2-(trimethylsilyloxy)ethanenitrile as the only detectable product. As shown in Fig. 4, the conversion of benzaldehyde over 10 mol% UiO-66-A increases gradually with time and reaches 96% within 46 h (Table 1, entry 4). By contrast, the blank reaction under the same conditions but in the absence of any catalyst led to only 17% conversion after 48 h (entry 1). These results clearly confirm the catalytic activity of UiO-66-A for cyanosilylation. To decide whether the observed catalytic activity is associated with the solid MOF or with leached species, a reaction with 10 mol% UiO-66-A was carried out under the same conditions. After 5 h, the solid was filtered off while hot, then the filtrate was again stirred and refluxed. GC analysis of the filtrate showed that almost no further reaction occurred even after 48 h (see Fig. 4). This control experiment clearly indicates that there are no active species leaching into the liquid phase and that catalysis of UiO-66-A is heterogeneous in nature.
2) in the presence of UiO-66-A yielded 2-phenyl-2-(trimethylsilyloxy)ethanenitrile as the only detectable product. As shown in Fig. 4, the conversion of benzaldehyde over 10 mol% UiO-66-A increases gradually with time and reaches 96% within 46 h (Table 1, entry 4). By contrast, the blank reaction under the same conditions but in the absence of any catalyst led to only 17% conversion after 48 h (entry 1). These results clearly confirm the catalytic activity of UiO-66-A for cyanosilylation. To decide whether the observed catalytic activity is associated with the solid MOF or with leached species, a reaction with 10 mol% UiO-66-A was carried out under the same conditions. After 5 h, the solid was filtered off while hot, then the filtrate was again stirred and refluxed. GC analysis of the filtrate showed that almost no further reaction occurred even after 48 h (see Fig. 4). This control experiment clearly indicates that there are no active species leaching into the liquid phase and that catalysis of UiO-66-A is heterogeneous in nature.
| Entry | Cat. | Quant.a (mol%) | Time | Conv.b (%) |
|---|---|---|---|---|
| a The quantity refers to the molar amount of Zr compared with that of benzaldehyde.b Conversion of benzaldehyde determined by GC using n-dodecane as the internal standard. Because only the addition product is formed in this reaction, the yield of the product is equal to the conversion of benzaldehyde.c The first cycle.d The second cycle.e The third cycle. | ||||
| 1 | — | — | 48 h | 17 |
| 2 | UiO-66-A | 2 | 48 h | 70 |
| 3 | UiO-66-A | 5 | 48 h | 85 |
| 4c | UiO-66-A | 10 | 46 h | 96 |
| 5 | UiO-66-A | 20 | 46 h | 94 |
| 6 | ZrO2 | 10 | 46 h | 51 |
| 7 | ZrCl4 | 10 | 46 h | 28 |
| 8d | UiO-66-A | 10 | 46 h | 90 |
| 9e | UiO-66-A | 10 | 46 h | 88 |
The effect of catalyst dose on the cyanosilylation conversion was also studied. As shown in Table 1 (entries 2–5), the conversion increases as the amount of the catalyst is increased from 2 to 10 mol%, but further increasing the amount above 10 mol% no longer leads to apparent improvement in conversion. The catalyst was compared with two easily available Zr(IV) reagents, ZrO2 and ZrCl4. The reaction performed in the presence of 10 mol% ZrO2 or ZrCl4 gave a much lower conversion (entries 6 and 7), suggesting that UiO-66-A is more efficient than the simple Zr compounds in promoting the reaction. This can be attributed to the microporous structure of UiO-66 and the uniformly distributed metal sites.
Several experiments were performed to check the recyclability of UiO-66-A (Table 1, entries 4, 8, 9). When the catalyst filtered out after the first run was used directly for the second run, the conversion of benzaldehyde was 71% within 46 h. The decreased activity may be mainly due to partial blocking of the pores. The presence of guest molecules in the pore is confirmed by the observation of the [220] peak in the XRD profile of the catalyst after the first reaction [see Fig. 2d]. The guest molecules can be removed by calcination at 300 °C for 2 h [Fig. 2e]. When the catalyst was calcinated after each run and then reused in the next run, the conversion of benzaldehyde remained at high levels (90 and 88% for the second and third run, respectively). The XRD profiles of the catalyst after each run (Fig. 2) suggest that the UiO-66 structure is essentially retained. The slight decrease in conversion may be because the repeated reaction and calcination procedures can cause minor damages to the framework, which however is undetectable by XRD.
The above results demonstrate that UiO-66 is a good heterogeneous catalyst for the addition of TMSCN to benzaldehyde. To check the generality, various carbonyl substrates were tested under given conditions. The results are collected in Fig. 5.
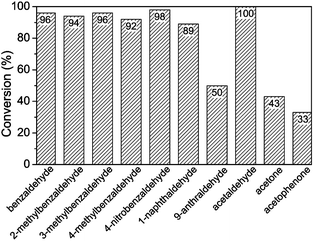 | ||
| Fig. 5 Conversion data for cyanosilylation of various substrates. Conditions: substrate (5 mmol), TMSCN (10 mmol), UiO-66-A (10 mol%), DCM (15 mL), 40 °C, 46 h. | ||
For substituted benzaldehydes under the same conditions, the conversion of 3-methylbenzaldehyde is very similar to that of benzaldehyde, while 2- and 4-methylbenzaldehydes show slightly lower conversions, and 4-nitrobenzaldehyde shows a slightly higher conversion. These results indicate (weak) electronic effects of the substituent groups. The strong electron-withdrawing nitro group could significantly increase the reactivity of the aldehyde group. The rather weak effect of nitro observed in this study could be due to the leveling effect of strong Lewis acidity of the Zr(IV) centre. The bulky substrate 1-naphthaldehyde shows a lower conversion, and the bulkier 9-anthraldehyde showed an even lower conversion (50%). The size effects suggest that the catalytic reaction occurs, at least partially, within the pores of the MOF.
The catalyst also shows high activity for cyanosilylation of acetaldehyde, but ketones such as acetone and acetophenone show much lower conversions (<50%). This just reflects the lower reactivity of ketones compared to aldehydes.
Comparison between UiO-66-A and -H: different acidity
The activity of UiO-66-A can be attributed to the Lewis acid sites generated upon thermal calcination at 300 °C. It has been demonstrated that the calcination treatment leads to dehydroxylation of the Zr6O4(OH)4 cluster and generates coordinatively unsaturated Zr sites.31,32,65 Additionally, it has also been evidenced that the real materials have naturally occurring defects related to linker deficiency. Owing to such defects, the material after calcination contains more unsaturated Zr sites and has a more open framework.35,36,63,64For comparison, the catalytic properties of the hydrous form of the material, UiO-66-H, were tested for the cyanosilylation of benzaldehyde. Unexpectedly, it turned out that UiO-66-H has comparable activity to UiO-66-A (Table 2, entries 1 and 5). It has been demonstrated elsewhere that the dehydroxylation of the Zr6O4(OH)4 cluster in UiO-66 starts at about 100 °C and is completed at 300 °C.33 Not subjected to high-temperature calcination, UiO-66-H should have a much lower density of open Zr sites (if any) than UiO-66-A. Thus the catalytic activity of UiO-66-H should have a different origin.
| Entry | Cat. | Time (h) | Conv. (%) |
|---|---|---|---|
| a Conditions: benzaldehyde (5 mmol), TMSCN (10 mmol), DCM (15 mL), solid catalyst (10 mol%), 40 °C.b UiO-66-A was stirred with water for 1 h, filtered out and then heated under air atmosphere at 120 °C for 5 h.c UiO-66-A was stirred with DMF for 1 h, filtered out and then heated under air atmosphere at 70 °C for 5 h.d UiO-66-A was stirred with aqueous acetic acid (HOAc) for 1 h, filtered out and then heated under air atmosphere at 70 °C for 5 h.e Before the catalytic test, UiO-66-H was subjected to repeated treatments with water (immersing the solid in water with stirring for 1 d, decanting the supernatant, and then repeating the immersing–decanting procedure for several times) until the pH of the supernatant is significantly increased to 4.7 or 5.5, filtered out and then dried in air at 70 °C for 5 h.f The water treated solid UiO-66-Hwater5.5 was stirred with aqueous HOAc (0.5 mol L−1) for 1 h, filtered out and then dried in at 70 °C for 5 h.g 0.5 mL HOAc was added to the reaction mixture containing the water treated solid UiO-66-Hwater5.5.h 0.5 mL HOAc was added to the reaction mixture in the absence of any solid catalyst. | |||
| 1 | UiO-66-A | 46 | 96 |
| 2 | UiO-66-Awaterb | 46 | 36 |
| 3 | UiO-66-ADMFc | 46 | 55 |
| 4 | UiO-66-AHOAcd | 46 | 99 |
| 5 | UiO-66-H | 46 | 97 |
| 6 | UiO-66-Hwater4.7e | 46 | 74 |
| 7 | UiO-66-Hwater5.5e | 46 | 53 |
| 8 | UiO-66-Hwater5.5–HOAcf | 46 | 68 |
| 9 | UiO-66-Hwater5.5/HOAcg | 27 | 98 |
| 10 | HOAch | 27 | 10 |
Two hypotheses can be forwarded. First, the O–H groups in the inorganic cluster may serve as weak Brønsted acid for catalysis. To clarify this possibility, UiO-66-A was soaked in water to recover the Zr6O4(OH)4 cluster and then dried in air. It proved that the resulting solid exhibits much lower catalytic activity (Table 2, entry 2) than both UiO-66-A and UiO-66-H. This result, on the one hand, indicates that the activity of UiO-66-A arises from open Zr sites, which, upon treatment with water, are diminished by re-hydroxylation of the cluster and/or by coordination of water molecules to defect-related Zr sites; on the other hand, the result rules out the possibility that the O–H groups is responsible for the high activity of UiO-66-H. Treating UiO-66-A with DMF also leads to dramatically reduced activity (entry 3), indicating that DMF is also disadvantageous to the catalytic activity by interacting with Zr sites.
The second hypothesis is that the acetic acid enclosed in the pores acts as a Brønsted acid to promote the reaction. The presence of acidic species in UiO-66-H is indicated by the fact that the supernatant obtained by immersing UiO-66-H in water shows pH <4 (typically, stirring 150 mg solid in 3 mL water for 1 h led to pH ∼3.8), and a further confirmative evidence is that the 1H NMR spectrum of the supernatant obtained by immersing UiO-66-H in D2O shows a signal of acetic acid (δ = 1.86, s) besides those of DMF (δ = 7.76, s, 1H; 2.84, s, 3H; 2.69, s, 3H). After repeated water treatments (immersing the solid in water with stirring for 1 d, decanting the supernatant, and then repeating the immersing–decanting procedure), the acid in UiO-66-H could be partially removed, as was indicated by the significant increase in the pH of the supernatant. The role of the acid in catalysis could be demonstrated by several control experiments. (i) While the water treated UiO-66-A catalyst shows much reduced catalytic activity as mentioned above, treating the catalyst with aqueous acetic acid does not reduce but (slightly) enhances the activity (Table 2, entry 4). This could suggest that the poisoning of the Lewis acid sites by water is compensated by the introduction of acetic acid. (ii) The activity of UiO-66-H decreases significantly as the amount of acetic acid in the catalyst is reduced by repeated water treatments (entries 5–7), but the activity can be regained by treating the water treated UiO-66-H with aqueous acetic acid (entry 8). (iii) Furthermore, when acetic acid was added into the catalytic reaction system with water treated UiO-66-H, the conversion of benzaldehyde increases dramatically (entry 9). The above results clearly demonstrate that acetic acid plays an important role in the catalytic performance of UiO-66-H. It is worth noting that homogeneous acetic acid in the absence of the solid catalyst leads to very low conversion of benzaldehyde (entry 10), implying that there is some cooperative effect between acetic acid and the UiO-66 framework in activating benzaldehyde.
The heterogeneity and recyclability of UiO-66-H were also checked. A control test revealed no further conversion in the filtrate after the catalyst was filtered out (Fig. 6a), confirming the heterogeneity. For recycling tests, the isolated catalyst after each run was washed with 5 mL DCM and dried in air. It proved that the catalyst can be reused without significant loss of activity (Fig. 6b).
 | ||
| Fig. 6 Conversion vs. time plots for reactions over UiO-66-H: (a) filtration test, (b) recycling tests. | ||
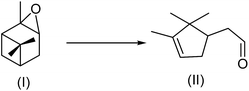
To further confirm that the catalytic activity of UiO-66-A and UiO-66-H is due to different acidity (Lewis or Brønsted acid), the isomerization of α-pinene oxide (I) to campholenic aldehyde (II) (Scheme 1) was used as a probe reaction. The isomerization can occurs in the presence of Lewis or Brønsted acids. Brønsted acids can lead to a mixture of compounds in low yield, the selectivity to II being usually not higher than 55%, while with Lewis acids the selectivity to II is usually higher, even reaching 85%.66–68 In our experiment, the reactions of 5 mmol α-pinene oxide in dichloroethane (20 mL) were performed at 60 °C in the presence of 0.5 mmol solid catalysts. The reactions were allowed to reach complete conversion. The selectivity to II remains almost unchanged during each reaction. The selectivity with UiO-66-H is about 45%, comparable to some Brønsted acid catalysts (such as the sulfonic resin Dowex 50Wx4-100) in the same solvent.66 Differently, the selectivity with UiO-66-A reaches 76%, well above the threshold value (55%) and comparable to the values previously reported for Cu-BTC and MIL-100-Fe (68–84%).21,66 These results clearly support the different origins of acidity in the UiO-66-A and UiO-66-H catalysts.
The effect of additives
In a control experiment to check if the Lewis acidity of UiO-66-A can be neutralized by a Lewis base, pyridine, which is a widely-used catalytic poison for metal sites,11,69 was added into the catalyst-DCM suspension before adding benzaldehyde and TMSCN. To our surprise, the addition of pyridine does not slow down but dramatically speeds up the cyanosilylation reaction. For instance, the reaction in the presence of 10 mol% UiO-66-A and 7.5 equivalent pyridine (referring to per Zr in the catalyst) almost reaches completion within 24 h, while the UiO-66-A catalyzed reaction under the same conditions but in the absence of pyridine needs more than 46 h to reach completion (Table 3, entries 1 and 2). The control experiment using the same amount of pyridine in the absence of UiO-66 gives a very small conversion of less than 6% (entry 10). The results suggest that pyridine alone does not have appreciable catalytic activity but serves as co-catalyst to promote the activity of UiO-66-A. Tentatively, the failure of pyridine in poisoning the Lewis acid sites may be because the space around Zr sites is too narrow (recalling that Zr6 clusters are at the corners of tetrahedral and octahedral cages) to allow for the coordination of a pyridine molecule. The coordination could be limited by the steric hindrance between the framework walls and the two pyridine C–H groups surrounding the nitrogen atom. However, the carbonyl oxygen atom of aldehydes does not suffer from such hindrance, so the space is enough for the coordination (activation) of carbonyl unless a rather bulky group is attached. The space is also enough for water and DMF to interact with the Zr sites, as suggested by the observation that the presence of water or DMF is disadvantageous to the catalytic reaction (vide supra). The much increased activity of the UiO-66-A-pyridine system compared with UiO-66-A or pyridine may be explained by a cooperative effect between isolated acid and base species (Zr sites and pyridine, respectively). The Lewis acid site can serve to activate the aldehyde by complexation with the carbonyl oxygen, while the Lewis base can activate TMSCN by hypercoordination to the silicon atom.48| Entry | Cat. | Additiveb | Time (h) | Conv. (%) |
|---|---|---|---|---|
| a Conditions: benzaldehyde (5 mmol), TMSCN (10 mmol), cat. (10 mol% if applied), DCM (15 mL), 40 °C; n.a. = not applied.b The amount of the additive is 7.5 equiv./Zr, if not specified in parentheses.c The number in parentheses is the yield of the cyanosilylation product. The main product is N-benzylidenebenzenamine. | ||||
| 1 | UiO-66-A | n.a. | 46 | 96 |
| 2 | Pyridine | 24 | 99 | |
| 3 | UiO-66-H | n.a. | 24; 48 | 65; 98 |
| 4 | Pyridine (3) | 24 | 84 | |
| 5 | Pyridine (6) | 24 | 94 | |
| 6 | Pyridine (7.5) | 24 | 94 | |
| 7 | Triethylamine | 0.5 | 100 | |
| 8 | Piperidine | 1 | 95 | |
| 9 | Aniline | 2 | 78 (16)c | |
| 10 | n.a. | Pyridine | 24 | <6 |
| 11 | Triethylamine | 0.5 | 100 | |
| 12 | Piperidine | 1 | 94 | |
| 13 | Aniline | 2 | 5 (2)c | |
Pyridine also promotes the catalytic activity of UiO-66-H. As can be seen from Table 3 (entries 4–6), the conversion of benzaldehyde over UiO-66-H increases as the amount of the co-catalytic pyridine additive increases from 0 to 6 equivalent, and further increasing the amount leads to no further increase in conversion.
Some other molecular additives have been tested for potential co-catalytic effects. Triethylamine (tertiary amine) and piperidine (secondary amine) themselves are efficient homogeneous catalyst for the reaction due to their strong Lewis basicity, and the high catalytic activities of the amine-UiO-66 combination can be due to the amine components (Table 3, entries 9, 10, 13, 14). Lewis base catalyzed cyanosilylation of aldehydes has been demonstrated elsewhere.48 Primary amines such as aniline can react with benzaldehyde, and the dominant product in the presence of aniline is the Schiff base N-benzylidenebenzenamine (Scheme 2; Table 3, entries 11 and 15). Notably, the yield of the Schiff base (3%) in the absence of UiO-66 is much lower than that (62%) with UiO-66. This suggests that UiO-66 could be a good catalyst for the Schiff-base condensation reaction of aldehydes with primary amines.
Conclusions
To summarize, we have performed a study using different forms of UiO-66 as porous solid catalysts for carbonyl cyanosilylation. As we desired, the anhydrous form (UiO-66-A), which has unsaturated metal sites in the Zr6 cluster, can catalyze the reactions of aldehydes with TMSCN in a heterogeneous and substrate-size selective way. It is rather unexpected that the hydrous form (UiO-66-H) shows comparable activity. With a number of intentionally designed control experiments, it has been shown that the acetic acid trapped in the framework during synthesis could be responsible for the activity of UiO-66-H, though acetic acid is inactive by itself. The different acidity in UiO-66-H (Brønsted acid) and UiO-66-A (Lewis acid) was probed and confirmed by the isomerization reaction of α-pinene oxide. Both UiO-66-H and UiO-66-A could be recycled without significant degradation in framework integrity and catalytic activity. Another unexpected finding of this study is that pyridine, which is inactive alone, acts as co-catalyst, rather than catalyst poison, to dramatically accelerate the catalytic reaction with UiO-66-H or UiO-66-A. This could be due to an acid–base synergistic mechanism. This work suggests that UiO-66 can be used as catalyst for cyanosilylation with no need of high-temperature activation. It represents an example where the nature of a MOF catalyst can be changed and the performance can be enhanced by the introduction of appropriate small molecules that are inactive when standing alone.Acknowledgements
This work is supported by the National Natural Science Foundation of China (NSFC Nos 21173083 and 21471057) and the Research Fund for the Doctoral Program of Higher Education of China.Notes and references
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- C. Wang, D. Liu and W. Lin, J. Am. Chem. Soc., 2013, 135, 13222 CrossRef CAS PubMed.
- Z. Zhang, Y. Zhao, Q. Gong, Z. Li and J. Li, Chem. Commun., 2013, 49, 653 RSC.
- M. P. Suh, H. J. Park, T. K. Prasad and D. W. Lim, Chem. Rev., 2012, 112, 782 CrossRef CAS PubMed.
- J. Della Rocca, D. M. Liu and W. B. Lin, Acc. Chem. Res., 2011, 44, 957 CrossRef CAS PubMed.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105 CrossRef CAS PubMed.
- P. Valvekens, F. Vermoortele and D. de Vos, Catal. Sci. Technol., 2013, 3, 1435 CAS.
- A. Dhakshinamoorthy, M. Opanasenko, J. Čejka and H. Garcia, Catal. Sci. Technol., 2013, 3, 2509 CAS.
- M. Yoon, R. Srirambalaji and K. Kim, Chem. Rev., 2012, 112, 1196 CrossRef CAS PubMed.
- A. Corma, H. Garcia and F. X. Llabres i Xamena, Chem. Rev., 2010, 110, 4606 CrossRef CAS PubMed.
- A. Dhakshinamoorthy, M. Alvaro, A. Corma and H. Garcia, Dalton Trans., 2011, 40, 6344 RSC.
- S. M. Cohen, Chem. Rev., 2012, 112, 970 CrossRef CAS PubMed.
- A. Dhakshinamoorthy, A. M. Asiric and H. Garcia, Chem. Soc. Rev., 2015, 44, 1922 RSC.
- A. Dhakshinamoorthy and H. Garcia, Chem. Soc. Rev., 2012, 41, 5262 RSC.
- H. R. Moon, D. W. Lim and M. P. Suh, Chem. Soc. Rev., 2013, 42, 1807 RSC.
- J. Juan-Alcañiz, J. Gascon and F. Kapteijn, J. Mater. Chem. A, 2012, 22, 10102 RSC.
- J. Gascon, A. Corma, F. Kapteijn and F. X. Llabrés i Xamena, ACS Catal., 2014, 4, 361 CrossRef CAS.
- S. H. Leenders, R. Gramage-Doria, B. de Bruin and J. N. Reek, Chem. Soc. Rev., 2015, 44, 433 RSC.
- Y. K. Hwang, D. Y. Hong, J. S. Chang, S. H. Jhung, Y. K. Seo, J. Kim, A. Vimont, M. Daturi, C. Serre and G. Ferey, Angew. Chem., Int. Ed., 2008, 47, 4144 CrossRef CAS PubMed.
- R. Srirambalaji, S. Hong, R. Natarajan, M. Yoon, R. Hota, Y. Kim, Y. Ho Ko and K. Kim, Chem. Commun., 2012, 48, 11650 RSC.
- A. Dhakshinamoorthy, M. Alvaro, H. Chevreau, P. Horcajada, T. Devic, C. Serre and H. Garcia, Catal. Sci. Technol., 2012, 2, 324 CAS.
- J. Gascon, U. Aktay, M. Hernandezalonso, G. Vanklink and F. Kapteijn, J. Catal., 2009, 261, 75 CrossRef CAS PubMed.
- J. Hermannsdorfer, M. Friedrich, N. Miyajima, R. Q. Albuquerque, S. Kummel and R. Kempe, Angew. Chem., Int. Ed., 2012, 51, 11473 CrossRef PubMed.
- B. Nepal and S. Das, Angew. Chem., Int. Ed., 2013, 52, 7224 CrossRef CAS PubMed.
- F. Salles, H. Jobic, A. Ghoufi, P. L. Llewellyn, C. Serre, S. Bourrelly, G. Ferey and G. Maurin, Angew. Chem., Int. Ed., 2009, 48, 8485 CrossRef PubMed.
- P. Horcajada, S. Surble, C. Serre, D. Y. Hong, Y. K. Seo, J. S. Chang, J. M. Greneche, I. Margiolaki and G. Ferey, Chem. Commun., 2007, 2820 RSC.
- L. Bromberg, X. Su and T. A. Hatton, Chem. Mater., 2013, 25, 1636 CrossRef CAS.
- A. Dhakshinamoorthy, M. Alvaro and H. Garcia, J. Catal., 2012, 289, 259 CrossRef CAS PubMed.
- O. V. Zalomaeva, A. M. Chibiryaev, K. A. Kovalenko, O. A. Kholdeeva, B. S. Balzhinimaev and V. P. Fedin, J. Catal., 2013, 298, 179 CrossRef CAS PubMed.
- Z. Zhang, L. Zhang, L. Wojtas, M. Eddaoudi and M. J. Zaworotko, J. Am. Chem. Soc., 2012, 134, 928 CrossRef CAS PubMed.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850 CrossRef PubMed.
- V. Guillerm, F. Ragon, M. Dan-Hardi, T. devic, M. Vishnuvarthan, B. Campo, A. Vimont, G. Clet, Q. Yang, G. Maurin, G. Ferey, A. Vittadini, S. Gross and C. Serre, Angew. Chem., Int. Ed., 2012, 51, 9267 CrossRef CAS PubMed.
- F. Vermoortele, R. Ameloot, A. Vimont, C. Serre and D. de Vos, Chem. Commun., 2011, 47, 1521 RSC.
- C. M. McGuirk, M. J. Katz, C. L. Stern, A. A. Sarjeant, J. T. Hupp, O. K. Farha and C. A. Mirkin, J. Am. Chem. Soc., 2015, 137, 919 CrossRef CAS PubMed.
- F. Vermoortele, B. Bueken, G. le Bars, B. van de Voorde, M. Vandichel, K. Houthoofd, A. Vimont, M. Daturi, M. Waroquier, V. van Speybroeck, C. Kirschhock and D. E. de Vos, J. Am. Chem. Soc., 2013, 135, 11465 CrossRef CAS PubMed.
- F. Vermoortele, M. Vandichel, B. van de Voorde, R. Ameloot, M. Waroquier, V. van Speybroeck and D. E. de Vos, Angew. Chem., Int. Ed., 2012, 51, 4887 CrossRef CAS PubMed.
- J. Kim, S.-N. Kim, H.-G. Jang, G. Seo and W.-S. Ahn, Appl. Catal., A, 2013, 453, 175 CrossRef CAS PubMed.
- F. G. Cirujano, A. Corma and F. X. Llabrés i Xamena, Chem. Eng. Sci., 2015, 124, 52 CrossRef CAS PubMed.
- M. J. Katz, S.-Y. Moon, J. E. Mondloch, M. H. Beyzavi, C. J. Stephenson, J. T. Hupp and O. K. Farha, Chem. Sci., 2015, 6, 2286 RSC.
- H. Fei, J. Shin, Y. S. Meng, M. Adelhardt, J. Sutter, K. Meyer and S. M. Cohen, J. Am. Chem. Soc., 2014, 136, 4965 CrossRef CAS PubMed.
- L. Shen, S. Liang, W. Wu, R. Liang and L. Wu, Dalton Trans., 2013, 42, 13649 RSC.
- J. Long, S. Wang, Z. Ding, S. Wang, Y. Zhou, L. Huang and X. Wang, Chem. Commun., 2012, 48, 11656 RSC.
- C. G. Silva, I. Luz, F. X. Llabres i Xamena, A. Corma and H. Garcia, Chem.–Eur. J., 2010, 16, 11133 CrossRef CAS PubMed.
- D. Sun, Y. Fu, W. Liu, L. Ye, D. Wang, L. Yang, X. Fu and Z. Li, Chem.–Eur. J., 2013, 19, 14279 CrossRef CAS PubMed.
- C. Wang, Z. Xie, K. E. deKrafft and W. Lin, J. Am. Chem. Soc., 2011, 133, 13445 CrossRef CAS PubMed.
- J.-J. Zhou, R. Wang, X.-L. Liu, F.-M. Peng, C.-H. Li, F. Teng and Y.-P. Yuan, Appl. Surf. Sci., 2015, 346, 278 CrossRef CAS PubMed.
- M. North, D. L. Usanov and C. Young, Chem. Rev., 2008, 108, 5146 CrossRef CAS PubMed.
- S. E. Denmark and W.-J. Chung, J. Org. Chem., 2006, 71, 4002 CrossRef CAS PubMed.
- S.-K. Tian, R. Hong and L. Deng, J. Am. Chem. Soc., 2003, 2003, 9900 CrossRef PubMed.
- M. Fujita, Y. J. Kwon, S. Washizu and K. Ogura, J. Am. Chem. Soc., 1994, 116, 1151 CrossRef CAS.
- C. Lv, C.-X. Miao, D. Xu, S. Wang, C. Xia and W. Sun, Catal. Commun., 2012, 27, 138 CrossRef CAS PubMed.
- F. Gandara, B. Gomez-Lor, M. Iglesias, N. Snejko, E. Gutierrez-Puebla and A. Monge, Chem. Commun., 2009, 2393 RSC.
- S. Neogi, M. K. Sharma and P. K. Bharadwaj, J. Mol. Catal. A: Chem., 2009, 299, 1 CrossRef CAS PubMed.
- M. K. Sharma, P. P. Singh and P. K. Bharadwaj, J. Mol. Catal. A: Chem., 2011, 342–343, 6 CrossRef CAS PubMed.
- S. Horike, M. Dinca, K. Tamaki and J. R. Long, J. Am. Chem. Soc., 2008, 130, 5854 CrossRef CAS PubMed.
- O. Ohmori and M. Fujita, Chem. Commun., 2004, 1586 RSC.
- P. Phuengphai, S. Youngme, P. Gamez and J. Reedijk, Dalton Trans., 2010, 39, 7936 RSC.
- R. F. D'Vries, M. Iglesias, N. Snejko, E. Gutierrez-Puebla and M. A. Monge, Inorg. Chem., 2012, 51, 11349 CrossRef PubMed.
- K. Schlichte, T. Kratzke and S. Kaskel, Microporous Mesoporous Mater., 2004, 73, 81 CrossRef CAS PubMed.
- A. Henschel, K. Gedrich, R. Kraehnert and S. Kaskel, Chem. Commun., 2008, 4192 RSC.
- A. Schaate, P. Roy, M. Wiebcke, A. Godt, P. Behrens, J. Lippke and F. Waltz, Chem.–Eur. J., 2011, 17, 6643 CrossRef CAS PubMed.
- S. J. Garibay and S. M. Cohen, Chem. Commun., 2010, 46, 7700 RSC.
- L. Valenzano, B. Civalleri, S. Chavan, S. Bordiga, M. H. Nilsen, S. Jakobsen, K. P. Lillerud and C. Lamberti, Chem. Mater., 2011, 23, 1700 CrossRef CAS.
- H. Wu, Y. S. Chua, V. Krungleviciute, M. Tyagi, P. Chen, T. Yildirim and W. Zhou, J. Am. Chem. Soc., 2013, 135, 10525 CrossRef CAS PubMed.
- A. M. Ebrahim, B. Levasseur and T. J. Bandosz, Langmuir, 2013, 29, 168 CrossRef CAS PubMed.
- L. Alaerts, E. Seguin, H. Poelman, F. Thibault-Starzyk, P. A. Jacobs and D. E. de Vos, Chem.–Eur. J., 2006, 12, 7353 CrossRef CAS PubMed.
- P. Kunkeler, J. van der Waal, J. Bremmer, B. Zuurdeeg, R. Downing and H. van Bekkum, Catal. Lett., 1998, 53, 135 CrossRef CAS.
- A. Corma and H. Garcia, Chem. Rev., 2003, 103, 4307 CrossRef CAS PubMed.
- A. Dhakshinamoorthy, M. Alvaro and H. Garcia, Chem.–Eur. J., 2010, 16, 8530 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |