Insights into the corrosion inhibition of copper in hydrochloric acid solution by self-assembled films of 4-octylphenol†
Abstract
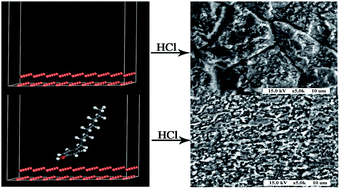
4-Octylphenol (OP) was used to form self-assembled films on copper to inhibit its corrosion in 0.5 M HCl solution. The corrosion protection ability of the OP films was evaluated using potentiodynamic polarization and electrochemical impedance spectroscopy studies. It was found that the highest inhibition efficiency was obtained when copper was self-assembled in a 1.0 mM OP solution for 24 h. The films on copper were characterized by scanning electron microscopy, contact angle tests, and Fourier transform infrared spectroscopy. Molecular simulations provided the adsorption model of OP molecules on the Cu (111) surface.

 Please wait while we load your content...
Please wait while we load your content...