An investigation of Pluronic P123–sodium cholate mixed system: micellization, gelation and encapsulation behavior†
Uzma Ashraf,
Oyais Ahmad Chat,
Masrat Maswal,
Suraya Jabeen and
Aijaz Ahmad Dar*
Department of Chemistry, University of Kashmir, Hazratbal, Srinagar-190 006, J&K, India. E-mail: aijaz_n5@yahoo.co.in; aijazdar@kashmiruniversity.ac.in; Fax: +91 1942414049; Tel: +91 9906417902
First published on 23rd September 2015
Abstract
The effect of sodium cholate (NaC) on the micellization and gelation characteristics of Pluronic P123 in aqueous media has been explored using tensiometry, rheology, dynamic light scattering (DLS), and densitometry. The aggregation characteristics were altered drastically with the addition of NaC as signified by an increase in the critical micelle concentration (CMC), critical micellization temperature (CMT) and critical gelation temperature (CGT). The results were explained on the basis of electrostatic and steric destabilization of P123 micelles by NaC. The apparent hydrodynamic diameter (DH) of P123 + NaC binary systems decreased upon addition of NaC up to 1.5 wt%. Further, in addition to an increase in DH, the presence of two types of scattering species was also evidenced with increase in NaC concentration from 2.5–10 wt%. The effect of NaC on the encapsulating capacity of P123 was also studied using naproxen and pyrene as two model hydrophobes. The work could give a sound understanding about the interaction and self-assembling behavior of Pluronics with the important physiological component i.e., bile salts which is important to consider in any pharmaceutical formulation involving Pluronics as drug delivery agents.
Introduction
Non-ionic amphiphilic triblock copolymers of type ABA with poly(propylene oxide) PPO as the hydrophobic central block and poly(ethylene oxide) PEO as the hydrophilic terminal blocks are commercially known as Pluronics or Poloxamers. Due to their amphiphilic character, the copolymer monomers can aggregate to form micelles above the critical micelle concentration (CMC) at a fixed temperature or above the critical micelle temperature (CMT) at a fixed concentration.1,2 With increase in temperature beyond CMT, the micelles formed undergo restructuring leading to an increase in their aggregation number, core size, and reduction in their degree of hydration. At still higher temperatures, large micellar clusters are formed before being phase separated at the cloud point (CP) forming two coexisting isotropic phases3–5 Moreover, with increase in concentration of copolymer well above its CMC, gels are formed and depending on the molecular architecture, molecular weight, concentration and temperature, such gels may assume cubic (face centered cubic (fcc), body centered cubic (bcc)), lamellar, and hexagonal crystalline structures.6 For example, F127 forms a cubic gel at concentrations below 50 wt% and at temperatures above 5 °C. The structures of P123 gels in the same concentration range are more complex, with cubic, hexagonal, and lamellar regions.7 It has also been observed that aqueous solutions of P123 show time dependent sphere-to-rod micellar growth on approaching their cloud points, the rate of micellar growth being dependent on temperature, heat cycling8 and nature of the electrolyte.9Pluronics have extensively attracted attention of researchers in view of their unique solution behaviour, core–shell aggregate formation, phase behaviour, rheological properties,10–15 dispersion stabilization, lubrication, low toxicity and minimal immune response16–18 rendering their immense applicative role in detergency, biomedical and pharmaceutical fields, tissue engineering, food protection, coating and painting, synthesis of different nanostructures etc.19–22 The properties of pluronics (rheological, colloidal, dispersion etc.) are known to get affected by the presence of additives. Numerous studies have been carried out to investigate the influence of a variety of additives such as electrolytes, non-electrolytes, organic solvents, homopolymers, hydrotropes and surfactants on the aggregation and gelation characteristics of Pluronics.23–32 Such studies are productive in optimizing the performance based properties of Pluronics for different applications due to their use in complex environments where the concomitant presence of many such additives is certain.
Understanding copolymer–surfactant interaction is essential not only from academic point of view but also from their high potential in industrial, biomedical and pharmaceutical applications.33 There are reports available in the literature where the interaction between various types of triblock copolymers and conventional surfactants like sodium dodecylsulfate (SDS), hexadecyltrimethylammonium bromide (HTAB), tetradecyltrimethylammonium bromide (TTAB), dimeric cationic surfactants, twin tailed and non-ionic surfactants have been investigated using various techniques.1,31,32,34–38 Several groups have explored the association mechanism and break up of Pluronic micelles upon addition of ionic surfactants by calorimetry, light scattering, cyclic voltammetry, time resolved fluorescence, and NMR techniques.1,31,32,37,38 Studies on the ionic surfactant-block copolymer mixed systems36,37 in aqueous media reveal that, depending on their concentrations and temperature, interactions between them lead to the formation of either mixed micelles or different type of mixed aggregates. Besides, the rheological properties of pluronics are significantly affected in presence of surfactants. Hetcht et al.37 studied the effect of adding SDS to 20–30 wt% aqueous gels of copolymer F127. As the SDS concentration was increased at a given copolymer concentration, the temperature range of the hard cubic gel became narrower and the gel eventually dispersed. Ganguly et al.31 studied the effect of adding SDS to 20–30 wt% solutions of copolymer P123 and reported the enhancement of gel formation and dispersion of the gel phase at SDS/P123 mole ratio of 2 and 5.5 respectively. The gelation temperature of F88 is observed to increase with the addition of SDS due to solubilization of SDS into F88 micelles.39
Bile acid/salts are known to possess hydrophilic concave α-face due to presence of multiple hydroxyl groups and the hydrocarbon like hydrophobic convex β-face. This facial amphiphilicity drives their self-aggregation to form micelles and other supramolecular networks.40–42 Bile salt micelles have been utilized as solubilizing media for various hydrophobic moieties with their solubilizing efficiency modulated by mixed micellization with other conventional surfactants.43 However, little emphasis has been given to the mixed micellization of bile salts with Pluronics. Very recently, Roy et al.44 investigated the role of NaTc (sodium taurocholate) and NaDc (sodium deoxycholate) on the self-assembled architecture of P123 micelles. The study revealed that the bile salts reside at the core–corona interface of the P123 micelle, thereby increasing the polarity of the bile salt–P123 mixed micelle. Also, the study evidenced the formation of two types of complexes at higher mole fractions of bile salt–P123 (>1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). In addition, Gomez et al.2,45 studied the interaction between Pluronics (F127/F68) and bile salt NaTDc (sodium taurodeoxycholate) at the oil–water interface with the emphasis on the role of Pluronics in delaying the lipid digestion. Furthermore, the effect of bile salts on the aggregation characteristics of Pluronics at both low and high concentration regimes i.e., micellization and gelation is still unexplored. It has been reported46–48 that the aggregation characteristics of OE based nonionic surfactants are significantly affected by the bile salts. In view of this, bile salt induced modification of Critical Micellization Temperature (CMT), Critical Gelation Temperature (CGT) and loading capability of Pluronic deserves attention as most of the pharmaceutical and pharmacological applications rely on the temperature dependence of self-assembly and its properties. A sound understanding of Pluronic–bile salt interactions and self-assembling behaviour could be of utmost importance to understand micellar stability of Pluronics in presence of the physiological components, and therefore promises to contribute to the efficient design of stable Pluronic based delivery systems with the desired drug carrying capacity. Therefore the main objective of the present study was to provide an insight on the effect of sodium cholate (NaC) on micellization, gelation and encapsulation behavior of model triblock biocompatible copolymer Pluronic P123, being widely used in pharmaceutical and cosmetic formulations, in aqueous media using tensiometry, rheology, dynamic light scattering, densitometry and spectrophotometric measurements.
3). In addition, Gomez et al.2,45 studied the interaction between Pluronics (F127/F68) and bile salt NaTDc (sodium taurodeoxycholate) at the oil–water interface with the emphasis on the role of Pluronics in delaying the lipid digestion. Furthermore, the effect of bile salts on the aggregation characteristics of Pluronics at both low and high concentration regimes i.e., micellization and gelation is still unexplored. It has been reported46–48 that the aggregation characteristics of OE based nonionic surfactants are significantly affected by the bile salts. In view of this, bile salt induced modification of Critical Micellization Temperature (CMT), Critical Gelation Temperature (CGT) and loading capability of Pluronic deserves attention as most of the pharmaceutical and pharmacological applications rely on the temperature dependence of self-assembly and its properties. A sound understanding of Pluronic–bile salt interactions and self-assembling behaviour could be of utmost importance to understand micellar stability of Pluronics in presence of the physiological components, and therefore promises to contribute to the efficient design of stable Pluronic based delivery systems with the desired drug carrying capacity. Therefore the main objective of the present study was to provide an insight on the effect of sodium cholate (NaC) on micellization, gelation and encapsulation behavior of model triblock biocompatible copolymer Pluronic P123, being widely used in pharmaceutical and cosmetic formulations, in aqueous media using tensiometry, rheology, dynamic light scattering, densitometry and spectrophotometric measurements.
Experimental
Materials
Triblock copolymer having average composition of (PEO)20(PPO)70(PEO)20 (denoted as P123) and nominal molar mass of 5600 g mol−1 was an Aldrich product. Sodium cholate (NaC), pyrene and naproxen were also Aldrich products and were used without further purification. Triple distilled water was used in the preparation of all solutions. The structure of the copolymer, bile salt and the model solubilizates used in the study are presented in Scheme 1.Solution preparation for rheological, dynamic light scattering (DLS) and density measurements
Stock solutions of Pluronic P123 and NaC were prepared and left to equilibrate overnight in a refrigerator at the temperature of ca. 6 °C. The following day P123 was mixed with NaC forming mixed systems containing constant copolymer concentration of 25 wt% and varying concentration of NaC (0.1, 0.5, 1, 1.5, 2.5, 5 and 10 wt% of NaC). The binary solutions were again left to equilibrate for at least one week prior to the rheological and DLS measurements.For CMT determination by densitometric measurements, total concentration of binary mixtures was fixed at 1 mM and mole fraction of P123![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaC varied as 1
NaC varied as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 3
9, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 5
7, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 7
5, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 9
3 and 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
Tensiometry
Surface tension measurements of Pure P123, NaC and their associated binary mixtures of varying mole fractions 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 2
9, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 3
8, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 5
7, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 7
5, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 8
3, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 9
2, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (P123
1 (P123![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
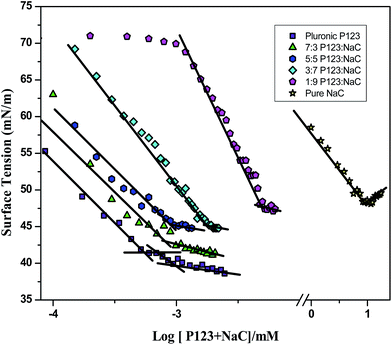
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaC) were carried out by the platinum ring detachment method with the Kruss K9 tensiometer equipped with a thermostable vessel holder. The total amphiphile concentration was varied by adding their concentrated stock surfactant solution (pure and binary systems) of known concentration in small installments using a Hamilton syringe to 20 mL water contained in the sample vessel placed in a vessel holder. Readings were taken after thorough mixing and temperature equilibration at 25 °C (±0.1 °C) by circulating water from a Brook field TC-102 thermostat through the vessel holder. The CMC values of all the surfactant solutions in pure and mixed states were determined from the plot of surface tension (γ) vs. logarithm of total amphiphile concentration (Fig. 1).
NaC) were carried out by the platinum ring detachment method with the Kruss K9 tensiometer equipped with a thermostable vessel holder. The total amphiphile concentration was varied by adding their concentrated stock surfactant solution (pure and binary systems) of known concentration in small installments using a Hamilton syringe to 20 mL water contained in the sample vessel placed in a vessel holder. Readings were taken after thorough mixing and temperature equilibration at 25 °C (±0.1 °C) by circulating water from a Brook field TC-102 thermostat through the vessel holder. The CMC values of all the surfactant solutions in pure and mixed states were determined from the plot of surface tension (γ) vs. logarithm of total amphiphile concentration (Fig. 1).
 | ||
| Fig. 1 Plot of Surface tension (γ) vs. logarithm of surfactant concentration for single and binary mixtures of P123 and NaC. | ||
Densitometry
The density measurements of triple distilled water, pure P123 and its binary mixtures with NaC containing their varying mole fractions but fixed total concentration of 1 mM were performed on Anton Paar DMA 4500 densitometer at varying temperatures ranging from 6 °C to 32 °C. The instrument employs an oscillating tube technique, where the relationship between the period of oscillation and the density is utilized. The fundamental frequency of the U-tube is a function of the systems mass making the oscillating frequency function of the sample density. The relation holds as long as the sample has a relatively low viscosity. From the measured density, the apparent partial specific volume of the binary mixtures were calculated using the following relation49
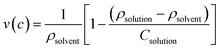 | (1) |
Rheometry
The rheological properties of the pure P123 solution and its binary mixtures with NaC were investigated by Anton Paar MCR-102 Rheometer equipped with a peltier system of temperature control with an accuracy of ±0.01 °C. Cone plate geometry (CP-50 diameter of 49.985 mm with cone angle of 1.006°) was used for the measurements. Test modes employed were: the shear rate sweep, frequency sweep and the temperature ramp. In steady shear experiments, shear rate was varied from 0.01 to 7000 s−1. Frequency sweep measurements were performed in angular frequency range of 0.1 to 500 rad s−1. All the experiments were carried out at 25 °C. However, the temperature was varied from 5–80 °C in the temperature ramp experiments at the fixed frequency of 1 Hz. At such a low frequency, it is easy to meet the requirements of linear viscoelasticity. Temperature was varied at a heating rate of 2 °C per minute. A small amount of low viscosity silicone oil was applied on the peripheral surface between the upper and lower plates to prevent evaporation of water.Dynamic light scattering
Size measurements were performed using Malvern Zetasizer Nano ZS90 dynamic light scattering instrument with 4 mW, 633 nm He–Ne laser. The angle of detector was set at 90°. Prior to dynamic light scattering measurements, solutions were filtered using 0.22 μm filter.Solubilization experiments
The solubility of two solubilizates viz. naproxen and pyrene was determined in separate experiments in pure P123 and P123 + NaC binary mixtures at their varying mole fractions and fixed total concentration of 1 mM. Excess amount of solubilizate was added to vials containing 1 mL of surfactant solutions (pure and binary) to ensure maximum solubility. The vials were then sealed with screw caps and agitated for a period of 24 hours on a magnetic stirrer at a temperature of 25 ± 0.5 °C. The solutions were subjected to centrifugation at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 rpm in order to remove any undissolved solubilizate. The concentration of the solubilized probes in different micellar solutions were then determined spectrophotometrically using Shimadzu Spectrophotometer (UV-1650PC) from their respective absorbance values following appropriate dilution of an aliquot of the supernatant with methanol. The solubility of naproxen and pyrene was determined at their characteristic wavelength of 332 nm and 334 nm respectively using the extinction coefficient of 2831.7 M−1 cm−1 for naproxen50 and 49 mM cm−1 for pyrene.51
400 rpm in order to remove any undissolved solubilizate. The concentration of the solubilized probes in different micellar solutions were then determined spectrophotometrically using Shimadzu Spectrophotometer (UV-1650PC) from their respective absorbance values following appropriate dilution of an aliquot of the supernatant with methanol. The solubility of naproxen and pyrene was determined at their characteristic wavelength of 332 nm and 334 nm respectively using the extinction coefficient of 2831.7 M−1 cm−1 for naproxen50 and 49 mM cm−1 for pyrene.51
Results and discussion
Mixed micelle formation between P123 and NaC
Representative plots of γ versus log[total amphiphile] for pure amphiphiles (P123 and NaC) and their mixtures at varying mole fractions are presented in Fig. 1. The CMC values are summarized in Table 1. The CMC value of NaC obtained is 9.9 mM which compares well with the literature value.52 The surface tension plot of pure P123 shows two breaks, where the high concentration break signifies the formation of multimolecular micelle and corresponds to the CMC.53 This is because the PEO-PPO-PEO copolymers are polydisperse both in mass and composition and hence causes the micellization process to occur gradually rather than at specific concentration. The CMC of P123 obtained is 0.00097 mM. However, the CMC values obtained for binary systems increases by increasing the mole fraction of NaC (Table 1) with appreciable increase at higher mole fractions of NaC. This could be probably because of the increased electrostatic and steric destabilization of the P123 micelles by NaC. Also, the experimental CMC values of binary systems were found to be less than the ideal CMC values calculated using Clint equation54 which indicates negative deviation from ideal behaviour for mixed micelle formation. The estimate of negative deviation and hence non ideality of binary surfactant systems has been quantified using Rubingh's model.55 The interaction parameter β, which accounts for deviation from ideality, is an indicator of degree of interaction between two surfactants in mixed micelle. β values along with the micellar mole fraction, XMi and activity coefficient fi, of the ith surfactant within mixed micelles calculated through Rubingh's equation55 are also presented in Table 1. A negative value of β indicates attractive interactions between the two surfactants in mixed micelles. In the binary mixtures of P123–NaC, negative β values of higher magnitude were observed indicating strong synergism between the two in the mixed micelle formation. This could be because of the reduction in the electrostatic self-repulsion of ionics and steric self-repulsion of non-ionics on mixing by dilution effect.56,57 Also, the strong synergism could be because of the participation of hydroxyl groups of NaC in hydrogen bonding with the POE groups present in the micellar corona. A large value of X1 indicates that the mixed micelles are rich in P123 component which is analogous to the results of ionic + non-ionic mixed surfactant systems where non-ionic surfactant is the dominating part in the mixed micelle.50 Due to the rigid structure of bile salts, their mole fraction in the mixed micelles is small in the whole mole fraction range.| P123–NaC | |||||
|---|---|---|---|---|---|
| α1 | cmcexp (cmcideal)/(mM) | γcmc (mN m−1) | β | XM1/XM2 | f1/f2 |
| a cmcideal = α1/cmc1 + α2/cmc2 for the ideal mixing as per the Clint equation where 1 and 2 represent two amphiphilic species. | |||||
| 1 | 0.00097 | 39.83 | |||
| 0.8 | 0.001 (0.0012) | 41.26 | −10.32 | 0.9051/0.0949 | 0.9112/0.0002 |
| 0.7 | 0.0011 (0.0013) | 41.96 | −10.14 | 0.8925/0.1075 | 0.8894/0.0003 |
| 0.5 | 0.00097 (0.0019) | 44.92 | −12.74 | 0.8063/0.1937 | 0.6201/0.0003 |
| 0.3 | 0.0019 (0.0032) | 44.99 | −10.63 | 0.8222/0.1778 | 0.7147/0.0008 |
| 0.1 | 0.005 (0.0096) | 47.5 | −9.79 | 0.791/0.209 | 0.6517/0.0022 |
| 0 | 9.9 | 48.4 | |||
The values of surface tension at the cmc (γcmc) of pure P123, NaC and their associated binary mixtures are also given in Table 1. γcmc value of pure P123 shows the lowest (39.8 mN m−1) value while as pure NaC shows the highest value of 48.4 mN m−1 indicating P123 to be more surface active than NaC. The γcmc values of binary mixtures increase progressively with increasing mole fraction of NaC showing that NaC decreased the surface activity of P123.
In order to evaluate the effect of NaC on the solubilization capacity of P123, we used P123 and its binary combinations with NaC to solubilize two model hydrophobes-naproxen and pyrene. The absorption spectra of naproxen and pyrene and their solubilized concentrations in P123 and P123 + NaC mixed systems are presented in Fig. 2. The solubility of both naproxen and pyrene decreased with increase in the mole fraction of NaC with more decrease in the solubility of latter compared to former. This could be because of the destabilization of P123 micelles by NaC owing to steric and electrostatic repulsions leading to formation of smaller sized mixed micelles as observed in other systems46 with decreased micellar core volume, thereby decreasing the solubility of hydrophobic compounds within the micellar core. The decreased hydrodynamic diameter of P123 micelles with the addition of NaC also indicates the formation of smaller sized micelles. Also, the incorporation of NaC P123 micelles is known to increase the hydrophillicity of the mixed micelle44 due to its significant charged interfacial area which could lead to the decreased solubilization of hydrophobic compounds. Since pyrene is more hydrophobic than naproxen, therefore it is expected to be solubilized in micellar core and hence a greater decrease in its extent of solubilization is observed. Naproxen, an acidic drug (pKa = 4.8) is reported to be solubilized in the interfacial region in case of non-ionic surfactants wherein the interaction between carboxyl groups of naproxen and PEO assists its solubilization.42 However, the incorporation of negative charge within the mixed micelle could have little effect on the solubilization of naproxen due to electrostatic repulsion as the micellar mole fraction of NaC in mixed micelle is very small. Therefore, the observed decrease in its solubility could be because of formation of small sized mixed micelles. The solubilization results therefore suggest that the change in micellar characteristics of P123 with the addition of NaC i.e., decreased micellar size and increased hydrophillicity of the mixed micelle is possibly responsible for reducing the solubility of naproxen and pyrene. Thus for the desired loading capacity, ratio of the two surfactants needs to be taken into consideration while formulating P123 based delivery vehicles.
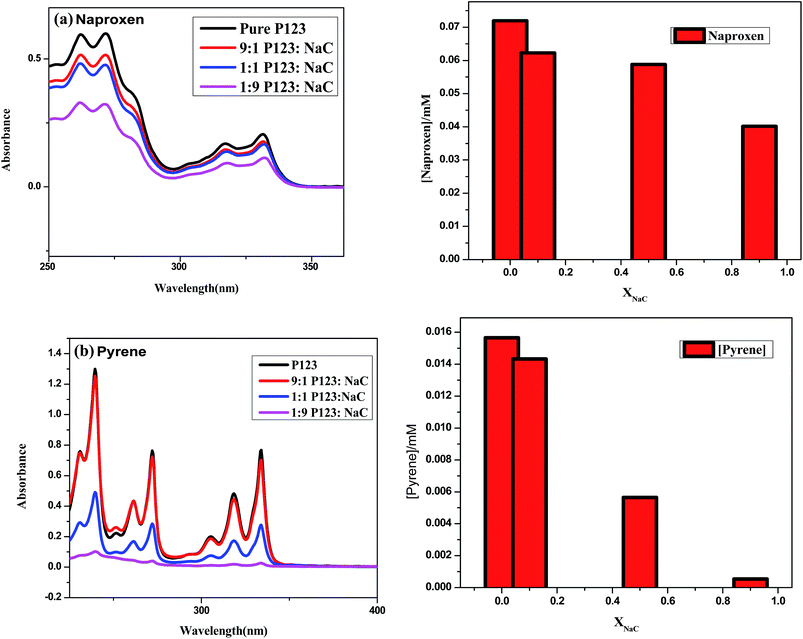 | ||
| Fig. 2 Absorbance vs. wavelength of (a) naproxen and (b) pyrene and their corresponding concentrations in P123 and P123 + NaC binary mixtures. | ||
Densitometric results
The density of the binary mixtures decrease with increase in temperature with a more profound decrease around a temperature which can be related to their critical micellization temperature (CMT).49 To probe this effect, apparent partial specific volume of binary mixtures were calculated using eqn (1). The variation of apparent partial specific volume v(c) of binary mixtures with temperature are presented in Fig. 3. The v(c) increases with temperature marking an abrupt increase at certain temperature called CMT which corresponds to the formation of micelles. The CMT of the binary mixtures increase with increase in the mole fraction of NaC. It increases from 18.97 °C in case of pure P123 to 22.91 °C in case of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 P123
9 P123![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaC indicating micellization of P123 is delayed by the addition of NaC similar to the tensiometric results wherein increase in CMC with the increase in mole fraction of NaC is observed. This can be ascribed to the formation of negatively charged mixed micelles due to the incorporation of NaC leading to electrostatic repulsion between the head groups. This consequently delays micellization and increases CMT of the binary mixtures. A similar suppression of micellization of F127 and F68 by sodium taurodeoxycholate in aqueous solution was reported by Gomez et al.2
NaC indicating micellization of P123 is delayed by the addition of NaC similar to the tensiometric results wherein increase in CMC with the increase in mole fraction of NaC is observed. This can be ascribed to the formation of negatively charged mixed micelles due to the incorporation of NaC leading to electrostatic repulsion between the head groups. This consequently delays micellization and increases CMT of the binary mixtures. A similar suppression of micellization of F127 and F68 by sodium taurodeoxycholate in aqueous solution was reported by Gomez et al.2
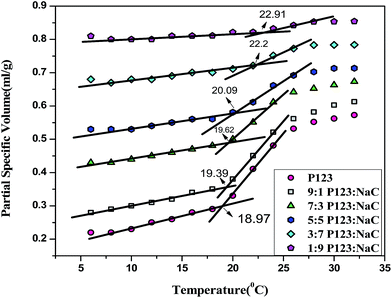 | ||
| Fig. 3 Plot of partial specific volume vs. temperature for Pure P123 and its binary mixtures with NaC. | ||
DLS results
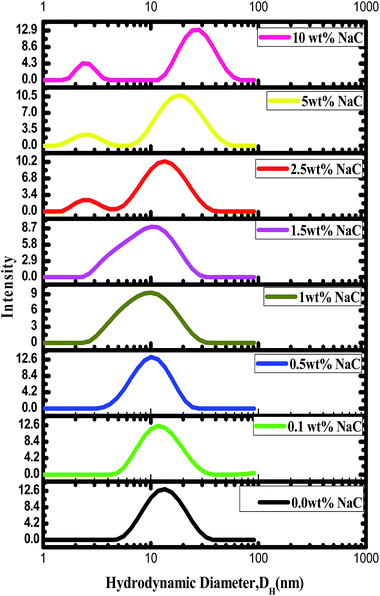
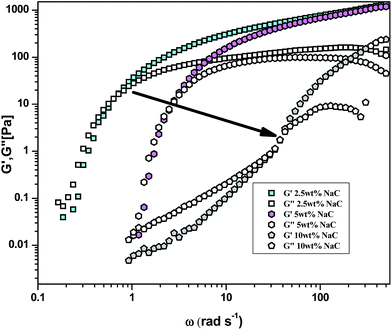
In order to investigate the influence of addition of NaC on the aggregation state of P123, DLS measurements were carried out on pure P123 (25 wt%) and its binary mixtures with varying concentration of NaC (0.1–10 wt%) at 25 °C. It is pertinent to mention that intensity fraction distribution of P123 is not affected much by increase in concentration from 5 to 25 wt% (Fig. S1†). Fig. 4 shows the intensity weighted size distribution of P123 (25 wt%) micelles and its binary combinations with NaC. The hydrodynamic diameter (DH) of pure P123 is found to be 14.3 nm which decreases to 10 nm with the addition of NaC upto concentration of 1.5 wt%. The formation of negatively charged mixed micelles with the addition of NaC to the already existing non-ionic P123 micelles results in the increase in intra aggregate repulsive forces favouring demicellization and hence decreasing micellar size.46 With further increase in concentration of NaC (from 2.5–10 wt%), DLS results evidenced the presence of two types of aggregates-smaller sized aggregates of DH = 2.6 nm and bigger aggregates with DH varying from 14.3 nm to 28.9 nm. At higher concentrations of NaC, mixed micelle does not allow further incorporation of NaC owing to enhanced electrostatic repulsions. However, the NaC added is expected to form NaC rich aggregate which may consist of cooperatively associated NaC interacting with copolymer unimers. This corresponds to the smaller sized aggregate of DH = 2.6 nm which is comparable to the size of pure NaC micelle.58 The larger aggregates correspond to the presence of copolymer rich aggregates resembling the corresponding pure copolymer micelle. Due to decreased contribution of NaC to the mixed micelles, larger copolymer rich aggregates with a wider size distribution are formed, indicating of increased polydispersity in their sizes. A similar observation has been reported by Roy et al.44 in bile salt–P123 systems at different mole ratios of bile salt–P123. Upto bile salt–P123 mole ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, size of P123 micelles decrease due to electrostatic repulsion in mixed micelle formation while as at higher mole ratios of bile salt–P123 (>1
3, size of P123 micelles decrease due to electrostatic repulsion in mixed micelle formation while as at higher mole ratios of bile salt–P123 (>1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), two types of complexes – copolymer rich P123–bile salt complexes and bile salt rich bile salt–P123 complexes are reported. Moreover, Jansson et al.59 also observed two types of copolymer–surfactant complexes in P123–SDS system at intermediate concentration of SDS. It is pertinent to mention that the time dependent micellar growth as observed by Ganguly et al.8 could not be envisaged in this study due to limited time range of the experiment.
3), two types of complexes – copolymer rich P123–bile salt complexes and bile salt rich bile salt–P123 complexes are reported. Moreover, Jansson et al.59 also observed two types of copolymer–surfactant complexes in P123–SDS system at intermediate concentration of SDS. It is pertinent to mention that the time dependent micellar growth as observed by Ganguly et al.8 could not be envisaged in this study due to limited time range of the experiment.
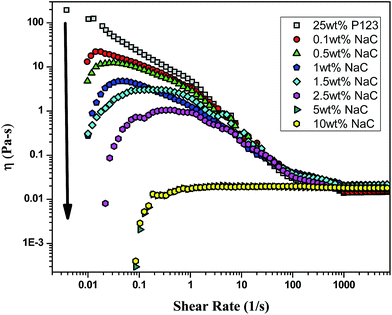
Rheological measurements
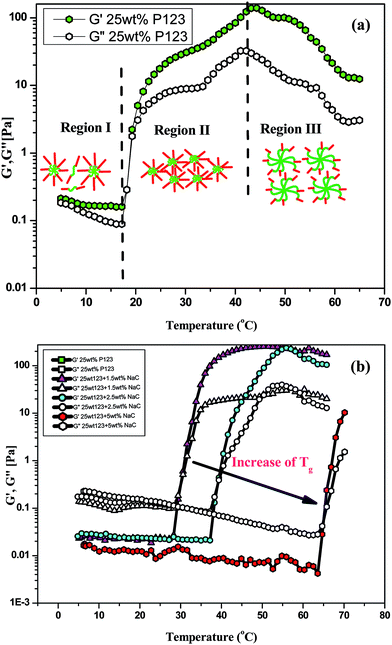 | ||
| Fig. 8 Variation of dynamic moduli of (a) 25 wt% P123 (b) P123 + NaC binary mixtures with temperature. | ||
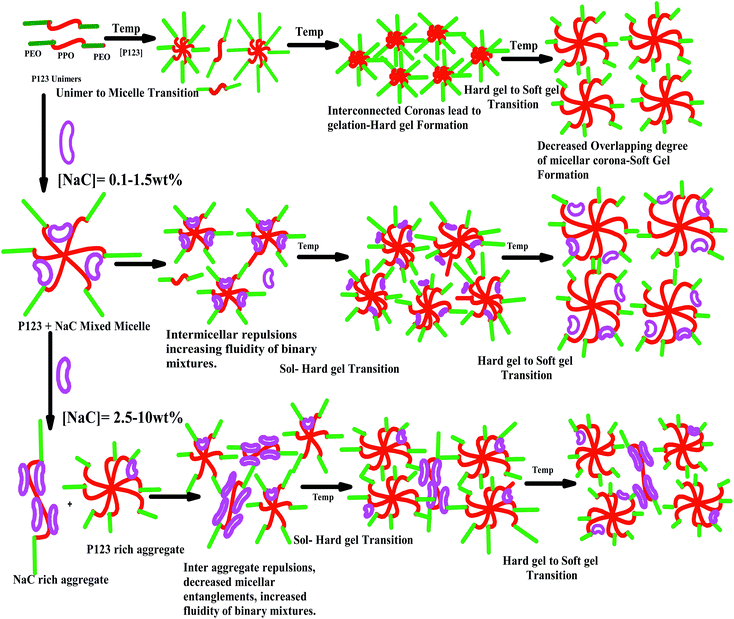 | ||
| Scheme 2 Schematic diagram representing effect of temperature and NaC on gelation characteristics of P123. | ||
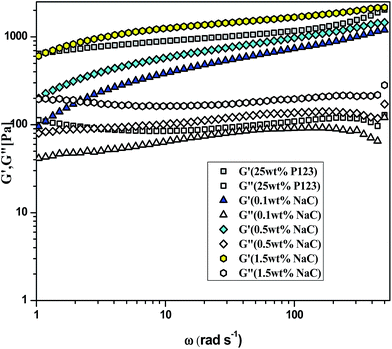
With the addition of NaC to 25 wt% P123, gelation is delayed to higher temperatures and the sol–gel transition temperature (Tg) increases with increase in NaC concentration [Fig. 8(b)]. Since gelation is not a process independent of micellization-depending on size of micelles, number of micelles and the possible bridges formed among micelles. Therefore, any factor which affects micellization is likely to affect gelation in a similar fashion. As mentioned earlier, addition of NaC delayed micellization of P123 or lead to drop of viscosity of the system, so does it lead to the dispersion of gel phase of P123 because of disruption of entanglements between the corona of mixed micelles due to intermicellar repulsion (Scheme 2). An increase of CMT of P123–NaC binary mixtures with increase in the mole fraction of NaC as observed from densiometric measurements for 1 mM concentration might have subsequently resulted in the increase of Tg which again corroborates the fact that the micellization and gelation are interdependent or directly related to each other. At the CMT, the micellar volume fraction is small as only a fraction of unimers form micelles. However, with increasing temperature above CMT, more and more unimers are converted to micelles leading to increase of micellar volume fraction which become sufficiently high for a gel to form at Critical Gelation Temperature (CGT). Also, the literature3–5 reports the increase of size or the aggregation number of the Pluronic based micelles with increasing temperature. At higher temperatures, the hydrophobicity of the P123 increases due to breaking of H-bonds of PPO unit which subsequently drives micellization and increases aggregation number leading to gelation. In presence of NaC, such micellar growth is suppressed due to increased charge repulsion, thus decreasing the temperature range for the stability of the gel phase. Also an increase in the size polydispersity of the micelles at higher mole fractions of NaC as revealed by DLS results would lead to lattice disruption and suppression of gelation as already speculated by Malmsten and Lindman.64
Conclusion
In conclusion, the micellar and gelation characteristics of P123 + NaC binary system has been investigated using tensiometry, rheology, densitometry and DLS measurements with the aim of obtaining useful information on the interaction and self-assembling behavior of facial amphiphile with the P123. The addition of NaC to P123 profoundly increased CMC, CMT and CGT which clearly indicates the suppression of micellization as well as gelation of 123. Since micellization and gelation are affected in a similar way, therefore, it confirms the interdependence of two processes. Also, the rheological studies show that the viscosity of P123 decreases dramatically with the addition of NaC and can be tuned to a specific value by the appropriate combination of two components. The rheological results were supported by DLS measurements. DLS measurements used to characterise the mixed micellar system confirm the size of mixed micellar aggregates first decreased with the addition of NaC upto 1.5 wt% and then two types of aggregates viz., small sized aggregates and larger aggregates are formed at higher concentration of NaC (2.5–10 wt%). The change in aggregation characteristics of P123 micelles by NaC resulted in decreased encapsulation capacity of the mixed aggregates. Thus, from the results it is evident that the aggregation and transport characteristics of P123 formulations are significantly influenced by the presence of biological amphiphiles. Therefore, it is imperative to take such effects into consideration while designing Pluronic based formulations.Acknowledgements
UA acknowledges the financial support from the University Grants Commision, India in the form of Senior Research Fellowship. AAD acknowledges Department of Science and Technology (DST), Govt. of India for providing funds under the FIST scheme to the Department of Chemistry, University of Kashmir for procuring various instruments.References
- J. S. Nambam and J. Philip, J. Phys. Chem. B, 2012, 116, 1499–1507 CrossRef CAS PubMed.
- A. T. Gomez, J. M. Valderrama, A. BelenJodar and T. J. Foster, Langmuir, 2013, 29, 2520–2529 CrossRef PubMed.
- G. Wanka, H. Hoffmann and W. Ulbricht, Colloid Polym. Sci., 1990, 268, 101–117 CAS.
- A. A. Al Saden, T. L. Whateley and A. T. Florence, J. Colloid Interface Sci., 1982, 90, 303–309 CrossRef CAS.
- Z. Zhou and B. Chu, J. Colloid Interface Sci., 1988, 126, 171–180 CrossRef CAS.
- S. Liu and L. Li, ACS Appl. Mater. Interfaces, 2015, 7, 2688–2697 CAS.
- C. Chaibundit, N. M. P. S. Ricardo, N. M. P. S. Ricardo, B. M. D. O'Driscoll, I. W. Hamley, S. G. Yeates and C. Booth, Langmuir, 2009, 25, 13776–13783 CrossRef CAS PubMed.
- R. Ganguly, M. Kumbhakar and V. K. Aswal, J. Phys. Chem. B, 2009, 113, 9441–9446 CrossRef CAS PubMed.
- Y. Kadam, R. Ganguly, M. Kumbhakar, V. K. Aswal, P. A. Hassan and P. Bahadur, J. Phys. Chem. B, 2009, 113, 16296–16302 CrossRef CAS PubMed.
- I. W. Hamley, J. Phys.: Condens. Matter, 2001, 13, R643–R671 CrossRef CAS.
- G. E. Newby, I. W. Hamley, S. M. King, C. M. Martin and N. J. Terrill, J. Colloid Interface Sci., 2009, 329, 54–61 CrossRef CAS PubMed.
- L. Zheng, C. Guo, J. Wang, X. Liang, S. Chen, J. Ma, B. Yang, Y. Jiang and H. Liu, J. Phys. Chem. B, 2007, 111, 1327–1333 CrossRef CAS PubMed.
- K. Mortensen and J. S. Pedersen, Macromolecules, 1993, 26, 805–812 CrossRef CAS.
- G. Wanka, H. Hoffmann and W. Ulbricht, Macromolecules, 1994, 27, 4145–4159 CrossRef CAS.
- Y. Liu, S. H. Chen and J. S. Huang, Macromolecules, 1998, 31, 2236–2244 CrossRef CAS.
- H. O. Ho, C. N. Chen and M. T. Sheu, J. Controlled Release, 2000, 68, 433 CrossRef CAS.
- S. Lee, R. Iten, M. Muller and N. D. Spencer, Macromolecules, 2004, 37, 8349 CrossRef CAS.
- S. Miyazaki, T. Tobiyama, M. Takada and D. Attwood, J. Pharm. Pharmacol., 1995, 47, 455–457 CrossRef CAS PubMed.
- I. W. Hamley, Block Copolymers in Solution, Wiley, Chichester, UK, 2005 Search PubMed.
- A. V. Kabanov, E. V. Batrakova and V. Y. Alakho, J. Controlled Release, 2002, 82, 189 CrossRef CAS.
- S. Krishnamoorthy, R. Pugin, J. Brugger, H. Heinzelmann, A. C. Hoogerwerf and C. Hinderling, Langmuir, 2006, 22, 3450–3452 CrossRef CAS PubMed.
- S. Alexander, T. Cosgrove, S. W. Prescott and T. C. Castle, Langmuir, 2011, 27, 8054–8060 CrossRef CAS PubMed.
- P. Bahadur, K. Pandya, M. Almgren, P. Li and P. Stilbs, Colloid Polym. Sci., 1993, 271, 657–667 CAS.
- E. B. Jorgensen and S. Hvidt, Macromolecules, 1997, 30, 2355–2364 CrossRef CAS.
- P. Alexandridis and J. F. Holzwarth, Langmuir, 1997, 13, 6074–6082 CrossRef CAS.
- Y. Cheng and C. Jolicoeur, Macromolecules, 1995, 28, 2665–2672 CrossRef CAS.
- J. Armstrong, B. Chowdhry, J. Mitchell, A. Beezer and S. Leharne, J. Phys. Chem., 1996, 100, 1738–1745 CrossRef CAS.
- Y. Kadam, B. Bharatiya, P. A. Hassan, G. Verma, V. K. Aswal and P. Bahadur, Colloids Surf., A, 2010, 363, 110–118 CrossRef CAS PubMed.
- D. Varade, R. Sharma, V. K. Aswal, P. S. Goyal and P. Bahadur, Eur. Polym. J., 2004, 40, 2457–2464 CrossRef CAS PubMed.
- J. Mata, T. Joshi, D. Varade, G. Ghosh and P. Bahadur, Colloids Surf., A, 2004, 247, 1–7 CrossRef CAS PubMed.
- R. Ganguly, V. K. Aswal, P. A. Hassan, I. K. Gopalakrishnan and S. K. Kulshreshtha, J. Phys. Chem. B, 2006, 110, 9843–9849 CrossRef CAS PubMed.
- N. V. Sastry and H. Hoffmann, Colloids Surf., A, 2004, 250, 247–261 CrossRef CAS PubMed.
- B. Jonsson, B. Lindman, K. Holmberg and B. Kronberg, Surfactants and Polymers in Aqueous Solution, John Wiley & Sons, Chichester, UK, 1998 Search PubMed.
- R. Kaur, S. Kumar, V. K. Aswal and R. K. Mahajan, Langmuir, 2013, 29, 11821–11833 CrossRef CAS PubMed.
- D. Löf, A. Niemiec, K. Schillen, W. Loh and G. Olofsson, J. Phys. Chem. B, 2007, 111, 5911–5920 CrossRef PubMed.
- M. Kumbhakar, J. Phys. Chem. B, 2007, 111, 14250–14255 CrossRef CAS PubMed.
- E. Hetcht, K. Mortensen, M. Gradzielski and H. Hoffmann, J. Phys. Chem., 1995, 99, 4866–4874 CrossRef.
- P. K. Singh, M. Kumbhakar, R. Ganguly, V. K. Aswal, H. Pal and S. Nath, J. Phys. Chem. B, 2010, 114, 3818–3826 CrossRef CAS PubMed.
- M. Vadnere, G. Amidon, S. Lindenbaum and J. L. Haslam, Int. J. Pharm., 1984, 22, 207 CrossRef CAS.
- P. P. Nair and D. Kritchevsky, The Bile Acids Chemistry, Physiology and Metabolism, Plenum Press, New York, 1973, vol. 2 Search PubMed.
- S. Mukhopadhyay and U. Maitra, Curr. Sci., 2004, 87, 1666–1683 CAS.
- R. G. Weiss and P. Terech, Molecular Gels: Materials with Self Assembled Fibrillar Networks, Springer, New York, 2006 Search PubMed.
- M. Maswal, O. A. Chat, S. Jabeen, U. Ashraf, R. Masrat, R. A. Shah and A. A. Dar, RSC Adv., 2015, 5, 7697–7712 RSC.
- A. Roy, N. Kundu, D. Banik, J. Kuchlyan and N. Sarkar, Phys. Chem. Chem. Phys., 2015, 17, 19977–19990 RSC.
- A. T. Gomez, J. M. Valderrama, A. B. J. Reyes, M. A. C. Vilchez and A. M. Rodriguez, Food Hydrocolloids, 2014, 34, 54–61 CrossRef PubMed.
- V. Patel, B. Bharatiya, D. Ray, V. K. Aswal and P. Bahadur, J. Colloid Interface Sci., 2015, 441, 106–112 CrossRef CAS PubMed.
- M. Maswal and A. A. Dar, Colloids Surf., A, 2013, 436, 704–713 CrossRef CAS PubMed.
- M. Posa, K. Popovic, D. Cirin and Z. Farkas, Fluid Phase Equilib., 2015, 396, 1–8 CrossRef CAS PubMed.
- P. Alexandridis, T. Nivaggioli and T. A. Hatton, Langmuir, 1995, 11, 1468–1476 CrossRef CAS.
- P. A. Bhat, G. M. Rather and A. A. Dar, J. Phys. Chem. B, 2009, 113, 997–1006 CrossRef CAS PubMed.
- R. Masarat, M. Maswal and A. A. Dar, J. Hazard. Mater., 2013, 244, 662–670 CrossRef PubMed.
- S. Reisa, C. G. Moutinho, C. Matos, B. de Castro, P. Gameiroc and J. L. F. C. Lima, Anal. Biochem., 2004, 334, 117–126 CrossRef PubMed.
- P. Alexandridis, V. Athanassiou, S. Fukuda and T. A. Hatton, Langmuir, 1994, 10, 2604–2612 CrossRef CAS.
- J. H. Clint, J. Chem. Soc., Faraday Trans., 1975, 171, 1327–1334 RSC.
- D. N. Rubingh, Mixed Micelle Solutions, in Solution Chemistry of Surfactants, ed. K. L. Mittal, Plenum Press, New York, 1979, pp. 337–354 Search PubMed.
- Q. Zhou and M. J. Rose, Langmuir, 2003, 19, 4555–4562 CrossRef CAS.
- W. Zhou and L. Zhu, J. Hazard. Mater., 2004, 109, 213–220 CrossRef CAS PubMed.
- J. SanthanalakshmI, G. Shanthalakshmi, V. K. Aswal and P. S. Goyal, Proc. - Indian Acad. Sci., Chem. Sci., 2001, 113, 55–62 CrossRef CAS.
- J. Jansson, K. Schillen, G. Olofsson, R. C. da Silva and W. Loh, J. Phys. Chem. B, 2004, 108, 82–92 CrossRef CAS.
- G. E. Newby, I. W. Hamley, S. M. King, C. M. Martin and N. J. Terrill, J. Colloid Interface Sci., 2009, 329, 54–61 CrossRef CAS PubMed.
- J. Jiang, C. Burger, C. Li, J. Li, M. Y. Lin, R. H. Colby, M. H. Rafailovich and J. C. Sokolov, Macromolecules, 2007, 40, 4016–4022 CrossRef CAS.
- J. Dey, S. Kumar, S. Nath, R. Ganguly, V. K. Aswal and K. Ismail, J. Colloid Interface Sci., 2014, 415, 95–102 CrossRef CAS PubMed.
- L. Li, H. Shan, C. Y. Yue, Y. C. Lam, K. C. Tam and X. Hu, Langmuir, 2002, 18, 7291–7298 CrossRef CAS.
- M. Malmsten and B. Lindman, Macromolecules, 1993, 26, 1282–1286 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra13002f |
| This journal is © The Royal Society of Chemistry 2015 |