Ultrathin tungsten oxide nanowires: oleylamine assisted nonhydrolytic growth, oxygen vacancies and good photocatalytic properties†
Abstract
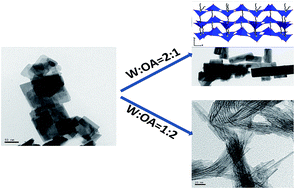
Ultrathin tungsten oxide nanowires with diameter of around 1.1 nm have been fabricated through a simple oleylamine (OA) assisted nonhydrolytic process. In the process, the amount of OA, reaction temperature and time impacted on controlling the formation of tungsten oxide ultrathin nanowires. At high W/OA molar ratios (>1 : 1), oriented attachment of OA molecules resulted in the preferential growth of nanowires along the [001] direction. At low W/OA molar ratios, small nanoparticles self-assembled into ultrathin nanowires. The ultrathin tungsten oxide nanowires exhibited a high photocatalytic activity for the degradation of Rhodamine B under visible light, likely due to their enhanced visible-light absorption and the presence of oxygen vacancies.

 Please wait while we load your content...
Please wait while we load your content...