9,10-Bis(N-alkylindole-3-yl-vinyl-2)anthracenes as a new series of alkyl length-dependent piezofluorochromic aggregation-induced emission homologues
Abstract
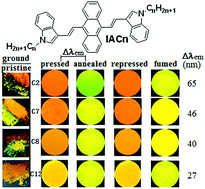
The introduction and length-extending of peripheral alkyl chains is usually to improve the solubility of conjugated organic molecules. This article reports the synthesis and optical properties of 9,10-bis(N-alkylindole-3-yl-vinyl-2)anthracenes (IACn) with different alkyl lengths. These homologues exhibit the strong alkyl length-dependent piezofluorochromic (PFC) behaviour, and their PFC spectral shifts are in the range of 27–65 nm. It is found that the shorter the N-alkyl length, the larger the spectral shift upon mechanical grinding or pressing. Wide-angle X-ray diffraction evidenced that the mechanical grinding has resulted in destruction of pristine ordered structures, formation of crystal defects, and appearance of some amorphous states, which is responsible for the PFC behaviour.

 Please wait while we load your content...
Please wait while we load your content...