Closoborate-transition metal complexes for hydrogen storage
Ravinder Kondaae,
Vijayanand Kalamseb,
Amol Deshmukhcd and
Ajay Chaudhari*e
aSchool of Physical Sciences, S. R. T. M. University, Nanded-431 606, India
bDepartment of Physics, S. G. G. S. I. E & T, Nanded-431 606, India
cMolecular Science and Technology Program, Taiwan International Graduate Program, Academia Sinica, Taipei 11529, Taiwan
dDepartment of Physics, National Central University, Jung-Li 32001, Taiwan
eDepartment of Physics, The Institute of Science, Fort, Mumbai-400 032, India. E-mail: ajaychau5@yahoo.com
First published on 11th November 2015
Abstract
We report hydrogen uptake capacity of early transition metal (TM) atom (Sc, Ti and V) decorated closoborate (B6H6) using density functional theory and second order Møller–Plesset method. Maximum of four hydrogen molecules can be adsorbed on B6H6Sc, B6H6Ti and B6H6V complex with their gravimetric hydrogen uptake of 6.51, 6.36, 6.21 wt% respectively. We have used M06, B3LYP and MP2 methods with 6-311++G** basis set for the study. The Gibbs free energy corrected adsorption energies show that adsorption of four H2 molecules on B6H6Ti and B6H6V is energetically favorable whereas it is unfavorable on B6H6Sc at 298.15 K at M06/6-311++G** and B3LYP/6-311++G** level. Many-body analysis approach has been used here to study the nature of interaction between adsorbed H2 molecules and the substrate and that between hydrogen molecules in a complex. The binding energy of B6H6Sc(4H2), B6H6Ti(4H2) and B6H6V(4H2) complex is found to be 39.44, 58.43 and 51.03 kcal mol−1 respectively using M06/6-311++G** level of theory. Interaction between inorganic material-metal complexes with adsorbed H2 molecules is found to be attractive for all the three complexes. The charge transfer between Ti and adsorbed H2 molecules is more than that for Sc and V. The HOMO–LUMO gap shows that all the three H2 adsorbed complexes are kinetically stable. The dimers of TM-closoborate complexes in head-to-tail type configuration and multi-transition metal atom decorated closoborate complexes have also been studied. In both the cases number of H2 molecules adsorbed per TM atom is not affected neither by dimerization nor multi-transition metal atom decoration.
1. Introduction
Due to deploying fossil fuels and increasing environmental issues such as global warming it has become necessary to search for a clean and more efficient energy source, its storage options and conversion technologies. Hydrogen is the most abundant element in the universe. It is also the most promising alternative to the carbon based fuels like gasoline. There are no hazardous emissions when it is used as an energy carrier. When used in fuel cells it gives water as the only byproduct. It is a light weight and ideal as a synthetic fuel. For using hydrogen as an energy carrier its production is not a problem but its storage in compact and lightweight package is a challenging task. For hydrogen economy to be a reality searching a material capable of storing hydrogen efficiently for vehicular applications is essential. However it is one of the most difficult challenges. As compared to bulk material nanoscale material and small molecules offer a higher energy storage capacity due to their unique properties such as high surface area, size confinement effect etc.1–4Several small organometallic complexes have been considered earlier from hydrogen point of view. It includes transition metal (TM)–acetylene,5–9 TM–ethylene,8,10–20 TM–CnHn rings and TM–CnHm complexes.21–30 There are several investigations on large inorganic structures as well for hydrogen storage in the form of hydrides. However hydrides require more energy to release hydrogen as it is stored in atomic form. In order to have the fast adsorption and desorption kinetics hydrogen should be stored in a molecular form. As compared to small organometallic complexes, inorganic material-metal (IOM) complexes have received fewer attentions as a hydrogen storage media. Few efforts are made earlier to study IOM complexes from hydrogen storage point of view.31–40
Hydrogen adsorption in B6H2Li2 has been studied by Srinivasu et al.31 and Pathak et al.32 They reported B6H2Li2 as a neutral system. In 1964 Boone has prepared the B6H6 ion which is the smallest member of hydroclosoborate family.41 Chang et al. have investigated the atomistic mechanisms governing hydrogen release and uptake processes in ammonia borane using the density functional theory (DFT) method.33 The spin-polarized DFT has been used to perform the simulations on 3d transition metal doped borazine.34 Hydrogen storage capacity of alkali metal, alkaline-earth metal and Ti decorated borazine has been investigated using DFT by Li et al.35 They concluded that the alkali metals bound strongly to the borazine. The H2 uptake capacity of 10.4 wt% was obtained for the Li decorated borazine. Using DFT and Møller–Plesset perturbation theory Pathak et al. have studied the hydrogen uptake of Li and Mg doped boranes.36 Structures and binding energies of hydrogen molecules adsorbed structures were calculated.
Hydrogen storage capacity of LimB6H6 (m = 1, 2) has been studied by Lu et al. using DFT and observed that the Li atom bound strongly to the substrate.37 Bandaru et al. have constructed the potential energy surface for the H2 release from ammonia borane with a novel bifunctional cationic ruthenium catalyst based on the sterically bulky β-diketiminato ligand using the DFT.38 Khan and Khan have performed the DFT calculations using hybrid B3PW91 functional to investigate hydrogen storage capacity of Ti, Cr, and Fe doped borazine.39 They concluded that the hydrogen storage capability of Ti–B3N3H6 in its quintet state is half than the triplet state and the quintet state is unfavourable for the hydrogen storage. Samolia and Kumar have studied the hydrogen trapping efficiency of a metal functionalized BN system at various high electron density sites using DFT at M05-2X/6-311G+(d) level of theory.40 Zhang et al. have studied Ti substituted boranes computationally for hydrogen storage applications.42 They predicted the hydrogen storage capacity of B5H5Ti and its TiH substituted analogue B4H4Ti2H2 as 8.6 and 10 wt% respectively with respective molecular hydrogen binding energy as −23.5 and −35 kJ mol−1 per H2 molecule. Kumar et al. have modified the metal–organic framework by incorporating transition metal through –OH and –SH functionalization of triphenyl linkers to improve the hydrogen storage capacity of metal–organic framework.43 They applied atoms in molecules theory to characterize the nature of interaction between H2 and metal centres. Their calculated interaction energy value per H2 varies from −5.47 to −15.67 kcal mol−1 and is suitable for room temperature hydrogen storage.
Most of the studies on hydrogen storage capacity of IOM complexes have been carried out either with alkali metal or alkaline earth metal doped inorganic material. However it has been observed that hydrogen adsorption on alkali or alkaline earth metal doped organic substrate is energetically unfavorable and these materials are not suitable as hydrogen storage media.7,9 This is true for wide range of temperature – room temperature, below as well as above room temperature. On the other hand early TM doped systems are good options for hydrogen storage. Therefore we have studied here hydrogen storage capacity of early TM doped inorganic material.
The aim of this work is to study hydrogen adsorption on TM–closoborate (TM = Sc, Ti and V) complexes using DFT and wave function method. The TM–closoborates considered here are B6H6Sc, B6H6Ti and B6H6V. Using Gibbs free energy corrected hydrogen adsorption energies we have suggested a temperature range over which H2 adsorption is possible on these complexes. Furthermore, the interaction energies between hydrogen molecules and IOM complexes as well as that between different hydrogen molecules in a complex are also studied.
2. Computational details
The geometry optimization of TMB6H6(nH2) (TM = Sc, Ti and V) complexes have been carried out using DFT with Becke's three parameter hybrid functional with LYP correlation functional (B3LYP),44–46 hybrid functional of Truhlar and Zhao (M06)47 and second order Møller–Plesset (MP2) method.48 We have used 6-311++G** basis set for all the calculations. All calculations were performed using Gaussian 09 suit of programs.49 Vibrational frequencies are also obtained at the same level of theory. Many-body analysis technique has been used to study nature of interaction between different molecules in hydrogen adsorbed complexes and contribution from many-body energies to the binding energy of a respective complex.50–53Many-body interaction energies
The decomposition of total energy of a complex can be written as
 | (1) |
| Δ2Ec(ij) = E(ij) − {E(i) + E(j)} | (2) |
| Δ3Ec(ijk) = E(ijk) − {E(i) + E(j) + E(k)} − {Δ2E(ij) + Δ2E(ik) + Δ2E(jk)} | (3) |
| Δ4Ec(ijkl) = E(ijkl) − {E(i) + E(j) + E(k) + E(l)} − {Δ2E(ij) + Δ2E(ik) + Δ2E(il) + Δ2E(jk) + Δ2E(jl) + Δ2E(kl)} − {Δ3E(ijk) + Δ3E(ijl) + Δ3E(ikl) + Δ3E(jkl)} | (4) |
Sum of relaxation energy, two-body energy, three-body energy, four-body energy, and five-body energy gives the binding energy of a complex. The BSSE corrected total energy can be calculated as suggested by Valiron and Mayer.54
3. Results and discussions
We have first optimized the geometries of B6H6TM (TM = Sc, Ti, V) complexes. The B6H6TM structure has six radially bonded H atoms attached to the B atoms at the vertices of an octahedron. The TM atoms Sc, Ti, V are counter-positioned on top of the face centered sites. Optimized structures of B6H6Sc(4H2), B6H6Ti(4H2) and B6H6V(4H2) at M06/6-311++G** level of theory are shown in Fig. 1. Maximum of four H2 molecules are adsorbed on B6H6Sc, B6H6Ti and B6H6V complex thereby showing their H2 uptake capacity of 6.51, 6.36, 6.21 wt% respectively. It is little higher than the target set by U. S. Department of Energy by 2015 (5.5 wt%). Structural parameters of H2 adsorbed complexes are presented in Table 1. The bond between B6H6 and transition metal is little elongated upon H2 adsorption for all the three cases. The elongation is more for B6H6V complex than B6H6Sc and B6H6Ti. Hydrogen molecules are adsorbed at shorter distance in B6H6V(4H2) than B6H6Sc(4H2) and B6H6Ti(4H2) complexes. | ||
| Fig. 1 Optimized structures of (a) B6H6Sc(4H2) (b) B6H6Ti(4H2) (c) B6H6V(4H2) at M06/6-311++G** level. | ||
| Assignments | B6H6Sc(4H2) | B6H6Ti(4H2) | B6H6V(4H2) |
|---|---|---|---|
| B6H6-TM | 2.29(2.32) | 2.23(2.25) | 2.25(2.28) |
| TM-1H2 | 2.21(2.25) | 2.03(2.06) | 1.83(1.83) |
| TM-2H2 | 2.26(2.30) | 2.04(2.13) | 1.98(1.99) |
| TM-3H2 | 2.24(2.25) | 1.91(1.93) | 1.81(1.84) |
| TM-4H2 | 2.14(2.17) | 1.91(1.92) | 1.87(1.94) |
Not only bond between B6H6 and transition metal but the H–H bond lengths of H2 molecules are also changed upon adsorption. The calculated H–H bond length for the isolated H2 molecule is found to be 0.750 Å at M06/6-311++G** level of theory. In B6H6Sc(4H2), B6H6Ti(4H2) and B6H6V(4H2) complexes the H–H bond lengths are in a range of 0.769–0.786 Å, 0.785–0.824 Å and 0.782–0.821 Å respectively. These H–H bond lengths are longer in H2 adsorbed complexes than that of free H2 molecule. It indicates that there is a Kubas interaction between transition metal atom and adsorbed H2 molecules.43 This elongation in H–H bond length than the free H2 molecule is due to the charge transfer from d orbital of the transition metal atom to the adsorbed H2 molecules.
Thermodynamic calculations have been carried out to evaluate the averaged H2 adsorption energy. The averaged adsorption energy with Gibbs free energy correction (ΔEG) for ‘n’ number of H2 adsorbed TM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B6H6 complex is calculated as:
B6H6 complex is calculated as:
| ΔEG = {EG[IOM] + nEG[H2] − EG[IOM(H2)n]}/n |
EG[X] and Gibbs free energy correction EG corr[X] are given by:
| EG[X] = E[X] + EG corr[X], and |
| EG corr[X] = Ezpe[X] + Ethm[X] + kBT − TS[X] |
Table 2 gives averaged adsorption energies without zero point energy correction (ΔE), with zero point energy correction (ΔEzpe) and with Gibbs free energy correction (ΔEG) at 298.15 K for B6H6TM(4H2) (TM = Sc, Ti, V) complexes at M06/6-311++G**, B3LYP/6-311++G** and MP2/6-311++G** levels. The Gibbs free energy corrected adsorption energies show that adsorption of four H2 molecules on B6H6Ti and B6H6V is energetically favorable whereas it is unfavorable on B6H6Sc at 298.15 K using M06 and B3LYP methods. Using MP2 method the adsorption of four H2 molecules on B6H6Sc and B6H6V complex is thermodynamically unfavorable where as it is favorable for B6H6Ti complex at room temperature. In order to find a temperature range over which H2 adsorption on these three complexes is energetically favorable, we have calculated Gibbs free energy corrected H2 adsorption energies for different temperatures. Temperature dependent ΔEG values are plotted in Fig. 2 using M06/6-311++G** and B3LYP/6-311++G** levels. As can be seen from Fig. 2 adsorption of four H2 molecules on B6H6Ti and B6H6V is energetically favorable for all the temperatures considered here at M06/6-311++G** as well as B3LYP/6-311++G** level of theory. Adsorption of four H2 molecules on B6H6Sc is energetically favorable below 270 K and 160 K at M06/6-311++G** and B3LYP/6-311++G** level respectively.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B6H6TM(4H2) (TM = Sc, Ti, V) complex at M06/6-311++G**, B3LYP/6-311++G** and MP2/6-311++G** levels of theory. All energies are in eV
B6H6TM(4H2) (TM = Sc, Ti, V) complex at M06/6-311++G**, B3LYP/6-311++G** and MP2/6-311++G** levels of theory. All energies are in eV
| Complex | M06/6-311++G** | B3LYP/6-311++G** | MP2/6-311++G** | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ΔE | ΔEzpe | ΔEG | ΔE | ΔEzpe | ΔEG | ΔE | ΔEzpe | ΔEG | |
| B6H6Sc(4H2) | 0.42 | 0.26 | −0.03 | 0.26 | 0.13 | −0.15 | 0.25 | 0.12 | −0.16 |
| B6H6Ti(4H2) | 0.63 | 0.45 | 0.16 | 0.55 | 0.36 | 0.07 | 0.57 | 0.39 | 0.09 |
| B6H6V(4H2) | 0.55 | 0.37 | 0.08 | 0.35 | 0.18 | 0.06 | 0.30 | 0.09 | −0.21 |
 | ||
| Fig. 2 Temperature dependent Gibbs free energy corrected H2 adsorption energy for B6H6Sc(H2)4, B6H6Ti(H2)4 and B6H6V(H2)4 at M06/6-311++G** and B3LYP/6-311++G** levels. | ||
For ideal hydrogen storage the H2 desorption from host material is necessary at ambient conditions. The H2 desorption temperature from B6H6Sc(4H2), B6H6Ti(4H2) and B6H6V(4H2) complexes is obtained using ΔEzpe and the van't Hoff equation55 as
TD = (ΔEzpe/kB)(ΔS/R − ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P)−1 P)−1 |
| Complex | M06/6-311++G** | B3LYP/6-311++G** | MP2/6-311++G** | |||
|---|---|---|---|---|---|---|
| (TD min) | (TD max) | (TD min) | (TD max) | (TD min) | (TD max) | |
| B6H6Sc(4H2) | 332 | 650 | 166 | 459 | 153 | 357 |
| B6H6Ti(4H2) | 574 | 1288 | 459 | 1186 | 497 | 2295 |
| B6H6V(4H2) | 472 | 956 | 230 | 383 | 115 | — |
Kinetic stability of H2 adsorbed complexes is verified by using a gap between Highest Occupied Molecular Orbital (HOMO) and Lowest Unoccupied Molecular Orbital (LUMO) at M06/6-311++G**, B3LYP/6-311++G** and MP2/6-311++G** levels. The HOMO–LUMO gap is plotted for different H2 adsorbed complexes in Fig. 3 at all three the levels of theories used here. The HOMO–LUMO gap for the maximum H2 adsorbed B6H6Sc and B6H6Ti complexes are higher than that for the respective isolated complexes (before H2 adsorption). It indicates more kinetic stability of H2 adsorbed B6H6Sc and B6H6Ti complexes than respective isolated IOM complex. There is negligible change in HOMO–LUMO gap for isolated B6H6V after maximum H2 adsorption on it. The stability of H2 adsorbed complexes is also verified by calculating vibrational frequencies for the three complexes. Table 4 shows selected vibrational frequencies and their assignment for H2 adsorbed complexes using M06 and B3LYP method. There are no imaginary frequencies for all the three complexes studied indicating that these complexes are quantum mechanically stable.
 | ||
| Fig. 3 HOMO–LUMO gap for B6H6Sc(H2)4, B6H6Ti(H2)4 and B6H6V(H2)4 at M06/6-311++G**, B3LYP/6-311++G** and MP2/6-311++G** levels. | ||
| Assignments | B6H6Sc(4H2) | B6H6Ti(4H2) | B6H6V(4H2) |
|---|---|---|---|
| B6H6-TM stretch (sym.) | 403(376) | 410(472) | 336(337) |
| TM-H stretch (sym.) | 544/563/511/685 | 590/693/859/930(934) | 958/999/873(915) |
| TM-H stretch (asym.) | 1183/1258(859/455) | 1236/1255/1449/1466(779/778) | 1546/1588/1322/1426 |
| H–H stretch | 3699/3772/3874//3937 | 3274/3314/3448/3644 | 3117/3267/3385/3722 |
| (4081/3918/4071/3851) | (3137/3293/3426/3500) | (3064/3382/3744/3897) |
Interaction energies between molecules
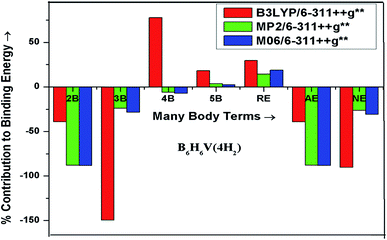
Many-body analysis technique has been used here to calculate various interaction energies for B6H6Sc(4H2), B6H6Ti(4H2) and B6H6V(4H2) complexes and are presented in Table 5 at M06/6-311++G** level. The % contribution from many-body energies, additive energy and nonadditive energy to the binding energy of B6H6Sc(4H2), B6H6Ti(4H2) and B6H6V(4H2) complexes is shown in Fig. 4, 5 and 6 respectively at all the three levels of theories used here. As can be seen from Table 4 total two-body energy has major contribution to the binding energy of a respective H2 adsorbed complex. The binding energy of B6H6Sc(4H2), B6H6Ti(4H2) and B6H6V(4H2) complex is found to be 39.44, 58.43 and 51.03 kcal mol−1 respectively at M06/6-311++G** level. This shows that each hydrogen molecule binds to the transition metal atom with energy in a range of 9.86 to 14.6 kcal mol−1 at M06/6-311++G** level of theory. It indicates that the complex considered here are suitable for hydrogen storage. The adsorption energies are within the range of physisorption and chemisorption and we expect fast adsorption and desorption kinetics.| Interaction terms | Interaction energy (kcal mol−1) | ||
|---|---|---|---|
| B6H6Sc(4H2) | B6H6Ti(4H2) | B6H6V(4H2) | |
| Two body | |||
| IOM–H1 | −9.98 | −14.63 | −15.20 |
| IOM–H2 | −11.67 | −18.73 | −10.08 |
| IOM–H3 | −9.56 | −21.47 | −14.07 |
| IOM–H4 | −14.16 | −21.59 | −12.92 |
| H1–H2 | 0.25 | 0.58 | 0.93 |
| H1–H3 | −0.11 | −0.05 | 0.42 |
| H1–H4 | −0.11 | −0.07 | 4.28 |
| H2–H3 | 0.31 | 3.34 | 1.21 |
| H2–H4 | 1.43 | 3.41 | −0.12 |
| H3–H4 | −0.12 | 0.16 | 0.58 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Three body | |||
| IOM–H1–H2 | 1.18 | 2.37 | −2.85 |
| IOM–H1–H3 | 2.64 | 2.58 | −4.02 |
| IOM–H1–H4 | 0.82 | 2.82 | −3.41 |
| IOM–H2–H3 | 1.13 | 0.13 | −1.22 |
| IOM–H2–H4 | 0.95 | 0.16 | 2.08 |
| IOM–H3–H4 | 1.17 | −0.33 | −3.74 |
| H1–H2–H3 | 0.03 | −0.32 | −0.69 |
| H1–H2–H4 | −0.06 | −0.26 | 0.06 |
| H1–H3–H4 | −0.02 | −0.10 | −0.71 |
| H2–H3–H4 | −0.01 | −0.50 | 0.04 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Four body | |||
| IOM–H1–H2–H3 | −2.40 | −1.87 | 0.24 |
| IOM–H1–H2–H4 | −0.95 | −2.05 | −2.52 |
| IOM–H1–H3–H4 | −1.33 | −0.41 | 0.92 |
| IOM–H2–H3–H4 | −1.19 | 1.18 | −2.37 |
| H1–H2–H3–H4 | −0.03 | −0.04 | −0.02 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Five body | |||
| IOM–H1–H2–H3–H4 | 1.54 | 1.04 | 2.51 |
| Sum of 2-body | −43.72 | −69.07 | −44.98 |
| Sum of 3-body | 7.83 | 6.56 | −14.45 |
| Sum of 4-body | −5.91 | −3.20 | −3.75 |
| Sum of 5-body | 1.54 | 1.04 | 2.51 |
| Relaxation energy | 0.82 | 6.25 | 9.65 |
| Additive energy | −43.72 | −69.07 | −44.98 |
| Non additive energy | 3.46 | 4.39 | −15.69 |
| Binding energy | −39.44 | −58.43 | −51.03 |
| BSSE corrected total energy | −918.05 | −1006.76 | −1101.28 |
Interaction of IOM complex with adsorbed H2 molecules is found to be attractive for all the three complexes. Total attractive contribution from IOM–Hi two-body interactions to the total two-body interaction energy is 103.8, 110.6 and 116.2% for B6H6Sc(4H2), B6H6Ti(4H2) and B6H6V(4H2) complex respectively. Total repulsive contribution from Hi–Hj interaction two-body energy terms to the binding energy of a respective complex is 3.8, 10.6 and 16.2%. As we go from B6H6Sc to B6H6V attractive% contribution from IOM–Hi terms and repulsive% contribution from Hi–Hj terms to the respective total two body energy increases. Though IOM–Hi two-body interaction energies are higher than Hi–Hj interaction energies the latter are not negligible.
Contribution from total three body energy to the binding energy of B6H6Sc(4H2), B6H6Ti(4H2) and B6H6V(4H2) is found to be 19.85 (repulsive), 11.22 (repulsive) and 28.31 (attractive)% respectively. Three-body energies for which IOM is one of the three body terms contribute about 100.8 (repulsive), 117.9 (repulsive) and 91 (attractive)% to total three body energy for B6H6Sc(4H2), B6H6Ti(4H2) and B6H6V(4H2) complex respectively. Contribution from Hi–Hj–Hk attractive interaction energy to total three-body energy for the B6H6Sc(4H2) complex is negligible (0.8%) whereas its contribution is 17.9 (attractive) and 9 (attractive)% for the B6H6Ti(4H2) and B6H6V(4H2) complex respectively. Most of the IOM–Hi–Hj three-body energies are repulsive in nature except the IOM–H3–H4 for B6H6Ti(4H2) and IOM–H1–H2, IOM–H1–H3, IOM–H1–H4, IOM–H2–H3 and IOM–H3–H4 for B6H6V(4H2) which are attractive in nature.
Similar to total two-body and total three-body energy, total four-body and total five-body energies also contribute significantly to the binding energy of respective complex. Total four-body energy has attractive contribution of 14.98, 5.47 and 7.34% to the binding energy of B6H6Sc(4H2), B6H6Ti(4H2) and B6H6V(4H2) complex respectively. Total five body energy contributes repulsively to the binding energy of respective complex and it is about 3.9, 1.8 and 4.9% for B6H6Sc(4H2), B6H6Ti(4H2) and B6H6V(4H2) complex respectively. In case of four-body energy the contribution from the four-body energy terms Hi–Hj–Hk–Hl is negligible as compared to IOM–Hi–Hj–Hk four-body energy terms. The % of contribution from terms containing IOM as one of the four-body terms is 99.3, 98.4 and 99.5% for B6H6Sc(4H2), B6H6Ti(4H2) and B6H6V(4H2) complex respectively.
Contribution from the relaxation energy to binding energy of respective complex is also not negligible. It has repulsive contribution of 2.1, 10.7 and 18.9% for B6H6Sc(4H2), B6H6Ti(4H2) and B6H6V(4H2) complex respectively at M06/6-311++G** level. Its contribution increases from B6H6Sc to B6H6V.
NBO analysis
Natural bond orbital (NBO)57 charges are calculated for all the complexes to understand their charge distribution during successive addition of H2 molecules. To get qualitative picture about charge transfer we calculated the charge on Sc, Ti, V atoms in B6H6Sc, B6H6Ti, B6H6V complexes with successive addition of H2 molecules using the NBO analysis. The charge on TM atom with successive addition of H2 molecules is plotted in Fig. 7 at M06/6-311++G** level. Charge on Sc and V atom is positive before H2 adsorption and remains positive even after adsorption of maximum four H2 molecules on B6H6Sc and B6H6V complex. Charge on Ti atom is also positive before adsorption of first H2 molecule on B6H6Ti and becomes negative after adsorption of four H2 molecules on it. It is observed that charge on Sc before first H2 adsorption is 1.07 and decreases with successive addition of H2 molecule up to third H2 molecule at M06/6-311++G** level. It slightly increases after addition of fourth H2 molecule. A large change in charge on Ti is observed after H2 adsorption. Charge on Ti before first H2 adsorption is 0.91 which becomes −0.54 after fourth H2 molecule adsorption. The charge on V decreases from 1.06 to 0.18 after adsorption of four H2 molecules. Charge transfer is more between Ti and adsorbed H2 molecules than that between Sc and H2 as well as V and H2. This is due to the fact that the Ti atom interacts strongly with the adsorbed H2 molecules than the Sc and V as can be seen from the higher two-body IOM–Hi interaction energies for B6H6Ti(4H2) system than that for B6H6Sc(4H2) and B6H6V(4H2) systems. More the charge transfer between transition metal atom and H2, more is the elongation in the H–H bond lengths. This is confirmed from more elongation in H–H bond lengths for adsorbed H2 molecules in B6H6Ti(4H2) complex than the B6H6Sc(4H2) and B6H6V(4H2) complexes. | ||
| Fig. 7 Charge on metal atom plotted as a function of number of adsorbed H2 molecules for B6H6Sc(nH2), B6H6Ti(nH2), B6H6V(nH2) (n = 1–4) complexes at M06/6-311++G** level. | ||
Dimerization of closoborate-TM complex and multi-TM decorated closoborate complexes
We have studied the dimers of closoborate-TM complexes to check whether there is a clustering of transition metal atoms. We have considered here head-to-tail type of dimers and the optimized geometries of one of the three dimers before and after H2 adsorption are shown in Fig. 8. As can be seen from Fig. 8 there is no clustering of transition metal atoms in case of head-to-tail type of structures for all the three cases before as well as after H2 adsorption. The number of H2 molecules adsorbed per TM atom remains four for all the three systems. However H2 molecules adsorbed on sandwiched TM atom are at little longer distance than that on TM atom which is decorated at one end. | ||
| Fig. 8 Head-to-tail type dimers of B6H6V (a) before H2 adsorption (b) after H2 adsorption at M06/6-311++G** level. | ||
We have also investigated multi-transition metal atom-closoborate complexes and their hydrogen storage capacity. Four transition metal atoms are decorated on closoborate for all the three cases. When four TM atoms are decorated on closoborate we do observed clustering of TM atoms. Fig. 9 shows one of the three structures with decoration of four Sc atoms on closoborate before and after H2 adsorption. Two pairs of transition metal atom dimers are formed in each case. The Sc–Sc, Ti–Ti and V–V atoms before (after) H2 adsorption are at a distance of 3.034 (3.154), 2.273 (3.069), 1.871 (2.936) Å from each other respectively in each pair. It indicates that TM–TM bond gets elongated upon H2 adsorption. Two pairs of Sc–Sc, Ti–Ti and V–V dimers are at a distance of 5.163, 4.880 and 4.528 respectively from each other in H2 adsorbed complexes. In single TM decorated closoborates, four H2 molecules are adsorbed per TM atom. Clustering of TM atoms does not affect the number of adsorbed H2 molecules per TM atom for all the three cases. Total number of H2 adsorbed on four TM atoms decorated closoborate is sixteen (four H2 per TM atom) for all the three cases. However two of the sixteen H2 molecules are dissociated and adsorbed in atomic form one on each TM dimer as shown in Fig. 9. Each TM dimer adsorbs eight H2 molecules one of which is dissociated.
 | ||
| Fig. 9 B6H6 decorated with four Sc atoms (a) before H2 adsorption (b) after H2 adsorption at M06/6-311++G** level. | ||
The averaged H2 adsorption energies for B6H6Sc4(16H2), B6H6Ti4(16H2) and B6H6V4(16H2) complexes without(with) zero point energy correction are found to be 0.52(0.36), 0.59(0.40) and 0.36(0.17) eV respectively. Similar to single TM atom decorated B6H6 complexes these energies are within the range of physisorption and chemisorption and we expect fast adsorption and desorption kinetics. The Gibbs free energy corrected adsorption energies for B6H6Sc4(16H2), B6H6Ti4(16H2) and B6H6V4(16H2) complexes are found to be 0.07, 0.10 and −0.13 respectively. It indicates that sixteen H2 molecule adsorption on four Sc and four Ti decorated closoborates is energetically favorable at ambient conditions whereas it is unfavorable on four V decorated closoborates. The binding energy of B6H6Sc4(16H2), B6H6Ti4(16H2) and B6H6V4(16H2) complex is found to be 195, 219 and 135 kcal mol−1 respectively at M06/6-311++G** level indicating that each hydrogen molecule binds to TM atom with energy in a range of 8.4 to 12.2 kcal mol−1. It indicates that not only single TM atom decorated B6H6 complex but four TM atom decorated B6H6 complexes are also suitable for hydrogen storage though clustering of TM atoms is observed for the latter.
4. Conclusions
We have studied hydrogen uptake capacity of transition metal decorated closoborate using M06, B3LYP and MP2 methods and 6-311++G** basis set. The number of hydrogen molecules adsorbed on each complex is the same. Contribution from many-body energies to the binding energy of a complex is studied. Many-body analysis for the three complexes reveals that not only two-body but higher many-body energies are also contributing significantly to the binding energy of respective complex. There are no soft modes for the three complexes indicating that these complexes are quantum mechanically stable. Thermochemistry calculations reveal that adsorption of four H2 molecules on B6H6Ti and B6H6V is energetically favorable for all the temperatures considered here whereas it is energetically favorable on B6H6Sc below 270 K at M06/6-311++G**. Minimum H2 desorption temperature for B6H6Sc(4H2), B6H6Ti(4H2), B6H6V(4H2) is found to be 332 K, 574 K and 472 K respectively whereas maximum desorption temperature for the respective complexes is 650 K, 1288 K and 956 K using M06/6-311++G** level of theory. Averaged adsorption energy is within the proposed range and affected by the functional employed in DFT calculations. In head-to-tail type of dimers of closoborate-TM complexes there is no clustering of TM atom observed and the number of H2 molecules adsorbed per TM atom remains four. H2 molecules adsorbed on sandwiched TM atom are at little longer distance than that on TM atom at one end. In case of four TM atoms decorated closoborate we do observed clustering of TM atoms. Number of H2 molecules adsorbed per TM atom remains the same with two of the sixteen H2 molecules are adsorbed in dissociated form.Acknowledgements
Financial support from C.S.I.R., New Delhi, India (Grant 03/1223) is gratefully acknowledged. We thank CDAC, Pune for providing Bioinformatics Resources and Applications Facility (BRAF).References
- http://www.eere.energy.gov/hydrogenandfuelcells/mypp.
- Solid state hydrogen storage materials and chemistry, ed. G. Walker, Woodhead Publ. Ltd., Cambridge, England, 2008 Search PubMed
.
- D. P. Broom, Hydrogen Storage Materials The Characterisation of Their Storage Properties, Springer, 2011 Search PubMed
.
- R. A. Varin, T. Czujko and Z. S. Wronski, Nanomaterials for solid state hydrogen storage, Springer, 2009 Search PubMed
.
- L. J. Ma, J. Jia, H. S. Wu and Y. Ren, Int. J. Hydrogen Energy, 2013, 38, 16185 CrossRef CAS
.
- M. Li-Juan, J. Jianfeng and H. S. Wu, Int. J. Hydrogen Energy, 2015, 40, 420 CrossRef
.
- V. Kalamse, N. Wadnerkar, A. Deshmukh and A. Chaudhari, Int. J. Hydrogen Energy, 2012, 37, 3727 CrossRef CAS
.
- V. Kalamse, N. Wadnerkar, A. Deshmukh and A. Chaudhari, Int. J. Hydrogen Energy, 2012, 37, 5114 CrossRef CAS
.
- P. Tavhare, V. Kalamse, R. Bhosale and A. Chaudhari, Struct. Chem., 2015, 26, 823 CrossRef CAS
.
- C. S. Liu and Z. Zeng, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 245419 CrossRef
.
- E. Durgun, S. Ciraci, W. Zhou and T. Yildirim, Phys. Rev. Lett., 2006, 97, 226102 CrossRef CAS PubMed
.
- W. Zhou, T. Yildirim, E. Durgun and S. Ciraci, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 085434 CrossRef
.
- N. Wadnerkar, V. Kalamse and A. Chaudhari, Theor. Chem. Acc., 2010, 127, 285 CrossRef CAS
.
- A. Chakraborty, S. Giri and P. K. Chattaraj, Struct. Chem., 2011, 22, 823 CrossRef CAS
.
- A. B. Phillips and B. S. Shivaram, Nanotechnology, 2009, 20, 204020 CrossRef CAS PubMed
.
- Y. Okamoto, J. Phys. Chem. C, 2008, 112, 17721 CAS
.
- N. He, T. Gao, Z. Zhang, X. Tian and H. Han, J. Mol. Struct.: THEOCHEM, 2009, 916, 147 CrossRef CAS
.
- V. Kalamse, N. Wadnerkar and A. Chaudhari, J. Phys. Chem. C, 2010, 114, 4704 CAS
.
- N. Wadnerkar, V. Kalamse, A. B. Phillips, B. S. Shivaram and A. Chaudhari, Int. J. Hydrogen Energy, 2011, 36, 9727 CrossRef CAS
.
- N. Wadnerkar, V. Kalamse and A. Chaudhari, RSC Adv., 2012, 2, 8497 RSC
.
- Z. Guo, W. Wu and H. Zhang, Struct. Chem., 2009, 20, 1107 CrossRef
.
- N. Wadnerkar, V. Kalamse and A. Chaudhari, Int. J. Hydrogen Energy, 2011, 36, 664 CrossRef CAS
.
- A. I. Martinez, J. Nano Res., 2009, 5, 113 CrossRef CAS
.
- P. F. Wecka and T. J. D. Kumar, J. Chem. Phys., 2007, 126, 094703 CrossRef PubMed
.
- A. B. Phillips, B. S. Shivaram and G. R. Myneni, Int. J. Hydrogen Energy, 2012, 37, 1546 CrossRef CAS
.
- B. Kiran, A. K. Kandalam and P. Jena, J. Chem. Phys., 2006, 124, 224703 CrossRef PubMed
.
- Q. Dong, W. Q. Tian, D. L. Chen and C. C. Sun, Int. J. Hydrogen Energy, 2009, 34, 5444 CrossRef CAS
.
- G. Chen, P. Jena and Y. Kawazoe, J. Chem. Phys., 2008, 129, 074305 CrossRef CAS PubMed
.
- N. Wadnerkar, V. Kalamse and A. Chaudhari, Struct. Chem., 2013, 24, 369 CrossRef CAS
.
- N. Wadnerkar, V. Kalamse, S. L. Lee and A. Chaudhari, J. Comput. Chem., 2012, 33, 170 CrossRef CAS PubMed
.
- K. Srinivasu and S. K. Ghosh, J. Phys. Chem. C, 2011, 115, 1450 CAS
.
- B. Pathak, K. Pradhan, T. Hussain, R. Ahuja and P. Jena, ChemPhysChem, 2012, 13, 300 CrossRef CAS PubMed
.
- K. Chang, E. Kim, P. F. Weck and D. Tománek, J. Chem. Phys., 2011, 134, 214501 CrossRef PubMed
.
- S. A. Shevlina and Z. X. Guo, Appl. Phys. Lett., 2006, 89, 153104 CrossRef
.
- M. Li, J. Li, Q. Sun and Y. Jia, J. Appl. Phys., 2010, 108, 064326 CrossRef
.
- B. Pathak, K. Pradhan, K. T. Hussain, R. Ahuja and P. Jena, ChemPhysChem, 2012, 13, 300 CrossRef CAS PubMed
.
- Q. L. Lu, J. W. Meng, W. J. Song and J. G. Wan, Int. J. Hydrogen Energy, 2013, 38, 13328 CrossRef CAS
.
- S. Bandaru, N. J. English, A. D. Phillips and J. M. D. MacElroy, J. Comput. Chem., 2014, 35, 891 CrossRef CAS PubMed
.
- M. S. Khan and M. S. Khan, Vacuum, 2014, 101, 151 CrossRef CAS
.
- M. Samolia and T. J. D. Kumar, RSC Adv., 2014, 4, 30758 RSC
.
- J. L. Boone, J. Am. Chem. Soc., 1964, 86, 5036 CrossRef CAS
.
- C. G. Zhang, R. Zhang, Z. X. Wang, Z. Zhou, S. B. Zhang and Z. Chen, Chem.–Eur. J., 2009, 15, 5910 CrossRef CAS PubMed
.
- R. M. Kumar, J. V. Sundar and V. Subramanian, Int. J. Hydrogen Energy, 2012, 37, 16070 CrossRef CAS
.
- A. D. Becke, Phys. Rev. A, 1988, 38, 3098 CrossRef CAS
.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS
.
- A. D. Becke, J. Chem. Phys., 1997, 107, 8554 CrossRef CAS
.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215 CrossRef CAS
.
- C. Møller and M. S. Plesset, Phys. Rev., 1934, 46, 618 CrossRef
.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, et al., Gaussian 09. Revision D.01, Gaussian Inc, Wallingford CT, 2009 Search PubMed
.
- S. S. Xantheas, J. Chem. Phys., 1994, 100, 7523 CrossRef CAS
.
- S. S. Xantheas, J. Chem. Phys., 1995, 102, 4505 CrossRef CAS
.
- A. Chaudhari, P. K. Sahu and S. L. Lee, J. Chem. Phys., 2004, 120, 170 CrossRef CAS PubMed
.
- A. Chaudhari and S. L. Lee, J. Chem. Phys., 2004, 120, 7464 CrossRef CAS PubMed
.
- P. Valiron and I. Mayer, Chem. Phys. Lett., 1997, 275, 46 CrossRef CAS
.
- W. Zhou, T. Yildirim, E. Durgun and S. Ciraci, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 085434 CrossRef
.
- D. R. Lide, CRC Handbook of Chemistry and Physics, New York, 75th edn, 1994 Search PubMed
.
- E. D. Glendening, A. E. Reed, J. E. Carpenter and F. WeinholdNBO Program, ver. 3.1, as implemented in link 607 of GAUSSIAN 09 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2015 |