Antibiofilm activity of tert-BuOH functionalized ionic liquids with methylsulfonate counteranions†
Abstract
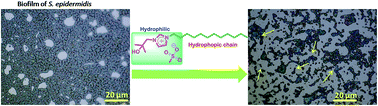
A series of varying alkyl chain length substituted tert-BuOH-functionalized-imidazolium mesylate salts [alkyl-tOHim][OMs] were synthesized and evaluated for antimicrobial activity and antibiofilm potential on selected pathogenic microorganisms including bacteria (Gram positive and Gram negative), yeast, and fungi. The dodecyl substituted ionic liquid [C12-tOHim][OMs] significantly prevented the biofilm formation of S. epidermidis at 100 µM concentration as well as showed noteworthy antimicrobial activity. We conclude that the ionic liquids (ILs) bearing chain lengths lower than the dodecyl length were found to be less effective against most of the tested pathogenic microorganisms.

 Please wait while we load your content...
Please wait while we load your content...