Carbonized polyaniline coupled molybdenum disulfide/graphene nanosheets for high performance lithium ion battery anodes†
Sheng Han‡
a,
Yani Ai‡a,
Yanping Tangb,
Jianzhong Jianga and
Dongqing Wu*ab
aSchool of Chemical and Environmental Engineering, Shanghai Institute of Technology, Shanghai 201418, China. E-mail: wudongqing@gmail.com
bSchool of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai 200240, China
First published on 6th November 2015
Abstract
Two-dimensional molybdenum disulfide/graphene hybrids wrapped in carbonized polyaniline (MCGs) have been constructed by the in situ growth of molybdenum trisulfide nanoparticles on polyaniline decorated graphene oxide nanosheets and the following carbonization. When the resulting hybrids were used as anode materials in lithium ion batteries, the carbonized polyaniline can effectively prevent the pulverization of MoS2 caused by the inner-plane volume expansion. It turns out that MCG-600, the sample thermally treated at 600 °C, manifests a capacity of 785 mA h g−1 after 300 cycles at the current density of 200 mA g−1 and a capacity of 724 mA h g−1 even at a high current density of 1 A g−1.
1. Introduction
Owing to extensive applications in portable electronics, electric vehicles and grid energy storage, lithium ion batteries (LIBs) with high energy density and long cycling life have aroused significant interest from both industry and academia.1,2 However, the electrochemical performances of current commercial LIBs are still retarded by the intrinsically low theoretical capacities of their graphite anodes (372 mA h g−1) and cannot fulfil the increasing demands for miniature LIBs with higher energy density.3–5 Therefore, the seeking of unprecedented anode materials with predominant Li storage capacity to replace graphite becomes an urgent task for material scientists.6–10 Compared with other candidates, the hybrids of carbon and inorganic nanoparticles have manifested overwhelming advantages as the alternatives of graphite anode. In these hybrids, the inorganic species with high theoretical capacities can provide plenty of active sites for lithium storage. On the other hand, the carbon components can ensure the conductivity of the whole electrode and prevent the structural damages caused by the volume expansion of inorganic nanoparticles.6,11–14To build up a hybrid material for LIB anode, molybdenum disulfide (MoS2), a layered transition-metal dichalcogenide with hexagonal arrays of Mo sandwiched between two S layers, is an appealing choice since it has both high theoretical capacity and favorable interlayer spacing for hosting Li atoms.15–19 As the carbon component, graphene with excellent conductivity and mechanism stability can effectively facilitate the migration of the electrons and relax the strain from the volume variation of MoS2.20–22 Nevertheless, only the inter-plane aggregation of MoS2 can be inhibited by two-dimensional (2D) graphene sheets. The inner-plane contact and pulverization of MoS2 on graphene still exist and will sabotage the long term stability of the electrode materials.23–25
Herein, we report the facile fabrication of the unprecedented 2D hybrids of carbonized polyaniline (PANI) coupled graphene and MoS2, named as MCGs, via the in situ growth of molybdenum trisulfide (MoS3) nanoparticles on the PANI decorated graphene oxide (GO) nanosheets and the following carbonization of the resulting ternary composites. The resulting hybrids possess well-defined 2D architectures with MoS2 nanoplates confined in the carbonized PANI. As the coupling linker between graphene and MoS2, the carbonized PANI can effectively improve the electrochemical performance of MCGs when they serve as the anode materials in LIBs. At a charging rate of 200 mA g−1, MCG-600, the sample thermally treated at 600 °C, can deliver an excellent specific capacity of 785 mA h g−1 after 300 charge/discharge cycles. Its capacity can be retained as 724 mA h g−1 even at an high current density of 1 A g−1.
2. Experimental
2.1. Materials
Sodium sulfide (Na2S), sodium molybdate (Na2MoO4), and aniline were obtained from Aladdin Reagent Co. Ltd. Sodium dodecyl benzene sulfate (SDBS), ammonium persulfate (APS), hydrochloric acid (HCl, 36 wt%), concentrated sulfuric acid (H2SO4, 98%), potassium permanganate (KMnO4), sodium nitrate (NaNO3), and hydrogen peroxide (H2O2) were bought from Sinopharm Chemical Reagent Co. Ltd. Unless otherwise stated, all the reagents were of analytical grade and used as received. All aqueous solutions were prepared with DI water.2.2. Preparation of PANI–GO sheets
GO was synthesized from natural graphite flakes by modified Hummers method. To prepare PANI functionalized GO nanosheets (PANI–GO), aniline (300 μL) were first mixed with SDBS (10 mg) in H2O (50 mL), and the solution was then added to the aqueous dispersion of GO (0.50 mg mL−1, 60 mL). The resulting mixture was ultra-sonicated for 15 min to form a homogeneous suspension. Subsequently, APS (0.7 g) dissolved in HCl (1 M, 50 mL) was added slowly to the suspension, which was vigorously stirred overnight at 0–5 °C. Then the green suspension of PANI–GO was obtained.2.3. Preparation of MoS3–PANI–GO hybrids
The obtained green suspension of PANI–GO was heated to 90 °C under stirring and Na2MoO4 (300 mg) dissolved in water (30 mL) was added dropwise. The mixture was continuously stirring at 90 °C for 3 h. Then, Na2S (1.5 g) dissolved in water (30 mL) was added to above mixture quickly, followed by vigorous stirring. After cooling down naturally, the resulting black suspension was centrifuged and washed with water and ethanol for several times to afford MoS3–PANI–GO.2.4. Preparation of MCGs
MoS3–PANI–GO was calcined in a tube furnace at 400, 600 or 800 °C with a heating rate of 5 °C min−1 for 3 h under N2 atmosphere to produce MCGs as the final products.2.5. Characterization
Scanning electron microscopy (SEM) measurements were performed on a FEI Sirion-200 field emission scanning electron microscope. Transmission electron microscopy (TEM) images were acquired using a Tecnai G2 F20 S-TWIN transmission electron microscope (FEI) operated at 200 kV. The sample was dispersed in ethanol and the suspension was dropped onto a copper grid covered with microgrid film. X-ray diffraction (XRD) analysis was performed on a D/Max-2500 X-ray diffractometer with Cu Kα radiation (λ = 0.1542 nm). FTIR spectra were recorded using Spectrum 100 spectrometer (Perkin Elmer, Inc., USA). Thermogravimetric analysis (TGA) curves were monitored on a Q5000IR apparatus (TA Instruments, USA). X-ray photoelectron spectroscopy (XPS) experiment was carried out on AXIS Ultra DLD system from Kratos with Al Kα radiation as X-ray source for radiation.2.6. Electrochemical measurements
Electrochemical tests of the hybrids as the anodes in lithium ion batteries (LIBs) were performed under ambient temperature using two-electrode 2032 coin-type cell. Pure lithium foil was used as the counter electrode. A microporous polypropylene membrane was used as the separator. The electrolyte consisting of a solution of 1 M LiPF6 in ethylene carbonate (EC)/dimethyl carbonate (DMC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume) was obtained from Ube Industries Ltd. The working electrodes were prepared by mixing the hybrids (MCG-400, MCG-600, or MCG-800), carbon black (Super-P), and poly(vinyl difluoride) (PVDF) at a weight ratio of 8
1 by volume) was obtained from Ube Industries Ltd. The working electrodes were prepared by mixing the hybrids (MCG-400, MCG-600, or MCG-800), carbon black (Super-P), and poly(vinyl difluoride) (PVDF) at a weight ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and pasting the mixture on pure copper foil (99.6%). The electrode was dried at 60 °C for 12 h in a vacuum oven and cut into sheets (Φ = 11 mm). The amount of active materials loaded on the electrode was ∼1.5 mg cm−2. The cells were assembled in an argon-filled glove box with the concentrations of moisture and oxygen below 1 ppm. Cyclic voltammogram (CV) test was conducted on a CHI 760 electrochemistry workstation at a scan rate of 0.1 mV s−1. AC impedance spectroscopy was obtained by applying a sine wave with amplitude of 1.5 V over the frequency range from 100 kHz to 0.01 Hz. Galvanostatic charge/discharge measurements were conducted on a battery tester (Land CA2001A) at various current rates in a potential range of 0.01–3.0 V vs. Li/Li+. The capacity was calculated based on the total mass of the active materials.
1 and pasting the mixture on pure copper foil (99.6%). The electrode was dried at 60 °C for 12 h in a vacuum oven and cut into sheets (Φ = 11 mm). The amount of active materials loaded on the electrode was ∼1.5 mg cm−2. The cells were assembled in an argon-filled glove box with the concentrations of moisture and oxygen below 1 ppm. Cyclic voltammogram (CV) test was conducted on a CHI 760 electrochemistry workstation at a scan rate of 0.1 mV s−1. AC impedance spectroscopy was obtained by applying a sine wave with amplitude of 1.5 V over the frequency range from 100 kHz to 0.01 Hz. Galvanostatic charge/discharge measurements were conducted on a battery tester (Land CA2001A) at various current rates in a potential range of 0.01–3.0 V vs. Li/Li+. The capacity was calculated based on the total mass of the active materials.
3. Results and discussion
The fabrication process of the 2D MCG hybrids is illustrated in Scheme 1. At first, GO coated with PANI (PANI–GO) were obtained by the polymerization of aniline in the suspension of GO. The resultant PANI–GO were then mixed with Na2MoO4 and Na2S to allow the in situ formation of MoS3–PANI–GO with MoS3 nanoparticles loading on the surface of the nanosheets. During this process, PANI can help the absorption of the inorganic precursors and provide confined spaces for the growth of MoS3 nanoparticles in case of the unwanted aggregations.26 To verify the role of PANI, MoS3–GO was also prepared in this work by the direct growth of MoS3 NPs on the surface of GO. Subsequently, the resulting MoS3–PANI–GO nanosheets were thermally treated at 400, 600 or 800 °C to generate MCGs as the final products. According to the pyrolysis temperatures, the samples are denoted as MCG-400, MCG-600 and MCG-800, respectively. In the thermal treatment, MoS3 is converted to MoS2 and GO can be thermally reduced. At the same time, nitrogen (N) doped carbon can be produced by the pyrolysis of PANI,27 which can serve as the protective shell for MoS2 and enhance the conductivity of the hybrids simultaneously.The morphologies and microstructures of MoS3–PANI–GO and MCGs were first investigated by field-emission scanning electron microscopy (FE-SEM) and transmission electron microscopy (TEM). The SEM images (Fig. 1 and S1†) indicate that MoS3–PANI–GO has typical 2D sheet-like morphology with the fully decoration of PANI particles (5–10 nm) on the surface. The presence of MoS3 NPs with the size of ∼2 nm can be observed in the TEM image of MoS3–PANI–GO (Fig. 1b). Without the confining effect of PANI, MoS3 NPs (having the diameters of ∼100 nm) manifest strong aggregation on the surface of MoS3–GO (Fig. S1†). As indicated by their SEM and TEM images, MCGs still keep the 2D architectures of their precursors after the thermal treatment (Fig. 1c, 2 and S2†). EDX and elemental mapping images further disclose that C, N, O, Mo and S elements are homogeneously distributed in MCG-600 (Fig. 1d). With the increase of the thermal treatment temperature, the edges of the MCG hybrids become smoother and the contrasts of the bright and dark areas are gradually weaker (Fig. 2), implying the reducing amount of the carbonized PANI components. These results are in accordance with the thermogravimetric analysis (TGA) results of the MCG hybrids (Fig. S3†).
 | ||
| Fig. 1 (a and b) SEM and (b) TEM images of MoS3–PANI–GO; (c) SEM image of MCG-600; (d) EDX with elemental mapping images of MCG-600. | ||
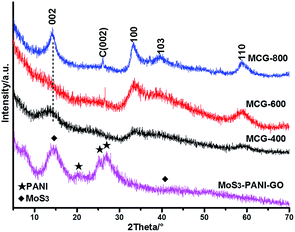
To evaluate the crystalline degree of the components in the hybrids, the X-ray diffraction (XRD) patterns of MoS3–PANI–GO, MCG-400, MCG-600 and MCG-800 were compared in Fig. 3. For MoS3–PANI–GO, two wide peaks at 14.5° and 42° are contribute to amorphous MoS3,28,29 and the peaks at 20°, 26.2° and 28° are from the crystallized PANI and graphene.30 In contrast, almost all of the diffractions in the XRD patterns of the MCGs can be indexed as the hexagonal phase MoS2 (JCPDS no. 37-1492),31 which confirms the conversion of MoS3 to MoS2 during the thermal treatment. Moreover, the intensities of the diffraction peaks from MoS2 gradually increase with the elevated thermal treatment temperature, indicative of the improved crystalline degree of the MoS2 components in the MCG hybrids.32
X-ray photoelectron spectroscopy (XPS) was further applied to analyze the chemical states of the elements in MoS3–PANI–GO and MCGs. As shown in Fig. 4a, C, N, O, Mo and S elements can be found in MoS3–PANI–GO and the calculated atomic ratio of Mo to S element is around 1 to 3, which further imply the existence of MoS3 in the composite.33–35 Further, as indicated by the XPS spectra of MCGs (Fig. S4†), all the hybrids contain C, N, O, Mo and S elements and the calculated atomic ratio of Mo to S element is 1 to 2, approaching the theoretical value of MoS2. Additionally the atomic contents of N in MCG-400, MCG-600 and MCG-800 are 13.35, 13.13, and 9.9%, respectively. Further, high-resolution N 1s spectra of MCG-600 discloses that N configurations with 22% graphite N (401.5 eV), 28% pyridic N (398.5 eV) and 50% pyrrolic N (400.2 eV) are presented in the hybrid (Fig. 4b).36
The combination of MoS2 and N-doped carbon from PANI in 2D graphene nanosheets makes the MCG hybrids attractive anode materials in LIBs. First, the electrochemical behavior of MCG-600 was investigated by cyclic voltammogram (CV) in the voltage range of 0.001–3 V at a scanning rate of 0.001 mV s−1. As shown in Fig. S5,† in the first cathodic scan, the peak at 1.67 V can be assigned to the intercalation of lithium into MoS2 to form LixMoS2,31 while the peak at 0.4 V is attributed to the reduction of LixMoS2 to Mo and Li2S.6,37 In the anodic scans, the peaks around 1.45 and 2.2 V can be associated with the conversions of Mo to MoS2 along with Li2S to polysulfide.31,37 In the subsequent cathodic scans, two reductive peaks appearing at 1.9 and 1.21 V should be derived from 2Li+ + S + 2e− → Li2S and MoS2 + xLi+ + xe− → LixMoS2, respectively.6,37 The electrochemical performances of MCGs were then evaluated by galvanostatic charge–discharge cycling at a current density of 100 mA g−1 in the voltage window of 0.01 to 3 V (Fig. 5a). The initial discharge and charge capacities of MCG-600 are 1554 and 1279 mA h g−1, respectively, corresponding to a coulombic efficiency (CE) of 82.3%. The irreversible loss in capacity is mainly due to electrolyte decomposition and the formation of a gel-like polymeric layer on the surface of electrode (solid electrolyte interface, SEI).37 The cycling performances of MCGs at a relatively high current density of 200 mA g−1 are then compared in Fig. 5b. Among the samples, MCG-400 only delivers a capacity of 315 mA h g−1 after 300 charge/discharge cycles. For the electrode based on MCG-600, its specific capacity can be kept as 785 mA h g−1 even after 300 cycles. In contrast, the capacity of MCG-800 is retained as 680 mA h g−1 under the same conditions.
Beside the excellent cycling stability, MCG-600 also exhibits the remarkable rate performance (Fig. 5c). Its specific capacities at 100, 200, 500, 1000, 2000, to 5000 mA g−1 are ∼1100, 939, 832, 724, 618 and 456 mA h g−1, respectively. And a reversible capacity of around 1100 mA h g−1 is restored when the current density is reset to 100 mA g−1. In contrast, MCG-800 and MCG-400 only have inferior capacities to MCG-600 at various current densities in the range of 100–5000 mA g−1.
The superior lithium storage performance of MCG-600 could be attributed to the synergistic effect of N-doped carbon from PANI, MoS2, and graphene. The N-doped carbon wrapping around MoS2 nanoplates can be utilized as the protective shell to buffer the volume expansion of MoS2 nanoplates during the charge/discharge process. On the other hand, the existence of N-doped carbon can also effectively improve the conductivity of the electrode. The MoS2 nanoplates confined in N-doped carbon from PANI can favorably provide highly accessible Li storage sites with both minimized insertion distance and interfacial storage.6 Moreover, the N atoms in the carbon framework of MCGs can both improve the electrolyte wettability of the electrode and provide extra active sites for the storage of lithium. Among the three samples, MCG-600 holds the balance between the content of N-doped carbon and the crystalline degree of MoS2 nanoplates and carbon frameworks, which endows it the outstanding electrochemical performance as the LIB anode. This can be confirmed by the electrochemical impedance spectra (EIS) of the three electrodes after 300 cycles. As shown in Fig. 5d, the diameter of semi-circle at high frequencies is remarkably reduced in the plot of MCG-600, compared with those of MCG-400 and MCG-800, indicating the greatly decreased charge-transfer resistance at the electrode/electrolyte interface due to the N-enriched carbon and graphene substrate in MCG-600.
4. Conclusions
In this work, a facile strategy towards carbonized PANI coupled MoS2/graphene nanosheets was developed. Among the three MCG hybrids, the one thermally treated at 600 °C manifests the best electrochemical performance as the anode in LIBs, which should be owing to the efficient combination of N-doped carbon, MoS2 and graphene. The present synthetic strategy can be further extended to the construction various 2D hybrids with promising applications in catalysis, sensors, and other secondary batteries.Acknowledgements
This work was financially supported by 973 program of China, (2014CB239701 and 2013CB328804), Natural Science Foundation of China (61235007, 61171037, and 21372155), Program of Professor of Special Appointment (Eastern Scholar), Shanghai Leading Academic Discipline Project (Project number 11SG54), ShuGuang Project, Shanghai Talent Development Funding (201335). We also thank the Instrumental Analysis Center of Shanghai Jiao Tong University for providing the relating measurements in this work.Notes and references
- A. Yoshino, Angew. Chem., Int. Ed., 2012, 51, 5798 CrossRef CAS PubMed.
- P. G. Bruce, S. A. Freunberger, L. J. Hardwick and J.-M. Tarascon, Nat. Mater., 2012, 11, 19 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. Morozov, D. Jiang, Y. Zhang, S. A. Dubonos, I. Grigorieva and A. Firsov, Science, 2004, 306, 666 CrossRef CAS PubMed.
- A. K. Geim, Science, 2009, 324, 1530 CrossRef CAS PubMed.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183 CrossRef CAS PubMed.
- C. Zhu, X. Mu, P. A. van Aken, Y. Yu and J. Maier, Angew. Chem., Int. Ed., 2014, 53, 2152 CrossRef CAS PubMed.
- Y. Hou, J. Li, Z. Wen, S. Cui, C. Yuan and J. Chen, Nano Energy, 2014, 8, 157 CrossRef CAS.
- H. Wang, H. Feng and J. Li, Small, 2014, 10, 2165 CrossRef CAS PubMed.
- K. Chang, Z. Wang, G. Huang, H. Li, W. Chen and J. Y. Lee, J. Power Sources, 2012, 201, 259 CrossRef CAS.
- P. G. Bruce, B. Scrosati and J. M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930 CrossRef CAS PubMed.
- L. Mai, X. Xu, C. Han, Y. Luo, L. Xu, Y. A. Wu and Y. Zhao, Nano Lett., 2011, 11, 4992 CrossRef CAS PubMed.
- H. S. Li, X. Y. Wang, B. Ding, G. Pang, P. Nie, L. F. Shen and X. G. Zhang, ChemElectroChem, 2014, 1, 1118 CrossRef CAS.
- L. Zhang and X. W. D. Lou, Chem.–Eur. J., 2014, 20, 5219 CrossRef CAS PubMed.
- X. Y. Yu, H. Hu, Y. Wang, H. Chen and X. W. D. Lou, Angew. Chem., Int. Ed., 2015, 54, 5331 CrossRef CAS PubMed.
- H. Ramakrishna Matte, A. Gomathi, A. K. Manna, D. J. Late, R. Datta, S. K. Pati and C. Rao, Angew. Chem., Int. Ed., 2010, 122, 4153 CrossRef.
- S. Wang, X. Jiang, H. Zheng, H. Wu, S.-J. Kim and C. Feng, Nanosci. Nanotechnol. Lett., 2012, 4, 378 CrossRef CAS.
- K. Chang and W. Chen, ACS Nano, 2011, 5, 4720 CrossRef CAS PubMed.
- L. Yang, S. Wang, J. Mao, J. Deng, Q. Gao, Y. Tang and O. G. Schmidt, Adv. Mater., 2013, 25, 1180 CrossRef CAS PubMed.
- H. Liu, D. Su, R. Zhou, B. Sun, G. Wang and S. Z. Qiao, Adv. Eng. Mater., 2012, 2, 970 CAS.
- S. Ding, J. S. Chen and X. W. D. Lou, Chem.–Eur. J., 2011, 17, 13142 CrossRef CAS PubMed.
- X. Zhou, L.-J. Wan and Y.-G. Guo, Nanoscale, 2012, 4, 5868 RSC.
- H. Jiang, D. Ren, H. Wang, Y. Hu, S. Guo, H. Yuan, P. Hu, L. Zhang and C. Li, Adv. Mater., 2015, 27, 3687 CrossRef CAS PubMed.
- K. Chang and W. Chen, Chem. Commun., 2011, 47, 4252 RSC.
- S. K. Das, R. Mallavajula, N. Jayaprakash and L. A. Archer, J. Mater. Chem., 2012, 22, 12988 RSC.
- J. Xiao, D. Choi, L. Cosimbescu, P. Koech, J. Liu and J. P. Lemmon, Chem. Mater., 2010, 22, 4522 CrossRef CAS.
- S. Li, D. Wu, C. Cheng, J. Wang, F. Zhang, Y. Su and X. Feng, Angew. Chem., Int. Ed., 2013, 125, 12327 CrossRef.
- H. Peng, Z. Mo, S. Liao, H. Liang, L. Yang, F. Luo, H. Song, Y. Zhong and B. Zhang, Sci. Rep., 2013, 3, 1765 Search PubMed.
- P. Afanasiev and I. Bezverkhy, Chem. Mater., 2002, 14, 2826 CrossRef CAS.
- X. C. Song, Y. Zhao, Y. F. Zheng and E. Yang, Adv. Eng. Mater., 2007, 9, 96 CrossRef CAS.
- Q. Gao, S. Wang, Y. Tang and C. Giordano, Chem. Commun., 2012, 48, 260 RSC.
- Y. Tang, D. Wu, Y. Mai, H. Pan, J. Cao, C. Yang, F. Zhang and X. Feng, Nanoscale, 2014, 6, 14679 RSC.
- S. Wang, Q. Gao, Y. Zhang, J. Gao, X. Sun and Y. Tang, Chem.–Eur. J., 2011, 17, 1465–1472 CrossRef CAS PubMed.
- T. Weber, J. Muijsers, J. van Wolput, C. Verhagen and J. Niemantsverdriet, J. Phys. Chem., 1996, 100, 14144 CrossRef CAS.
- J. Iranmahboob, S. D. Gardner, H. Toghiani and D. O. Hill, J. Colloid Interface Sci., 2004, 270, 123 CrossRef CAS PubMed.
- Y. H. Chang, C. T. Lin, T. Y. Chen, C. L. Hsu, Y. H. Lee, W. Zhang, K. H. Wei and L. J. Li, Adv. Mater., 2013, 25, 756 CrossRef CAS PubMed.
- Y.-H. Chang, R. D. Nikam, C.-T. Lin, J.-K. Huang, C.-C. Tseng, C.-L. Hsu, C.-C. Cheng, C.-Y. Su, L.-J. Li and D. H. Chua, ACS Appl. Mater. Interfaces, 2014, 6, 17679 CAS.
- S. Han, Y. Zhao, Y. Tang, F. Tan, Y. Huang, X. Feng and D. Wu, Carbon, 2015, 81, 203 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra12750e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |