Cellulose fatty acid esters as sustainable film materials – effect of side chain structure on barrier and mechanical properties†
Abstract
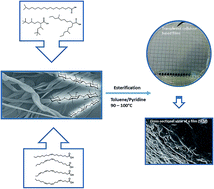
Cellulose is mainly utilized by industry for paper and packaging materials. Due to ecological awareness this biopolymer has recently received an increasing amount of attention as a renewable alternative for replacing traditional oil based products. In this work, hydrophobic cellulose based materials were prepared by acylation of cellulose with tall oil fatty acid based saturated, unsaturated and branched fatty acids. Films were prepared by casting, and their oxygen and water vapour permeabilities as well as mechanical and thermal properties were characterized. Unsaturation and branching had a significant effect on the properties of the films. Comparing these materials with already existing commercial products showed that fatty acid modification of cellulose yields films with increased thermal stability, low water vapour transmission rates and enhanced tensile and elastic properties.

 Please wait while we load your content...
Please wait while we load your content...