Effect of ionic strength on phosphorus sorption in different sediments from a eutrophic plateau lake
Wei Huangab,
Ying Lub,
Jihua Lib,
Zheng Zhengb,
Jibiao Zhangb and
Xia Jiang*a
aState Key Laboratory of Environmental Criteria and Risk Assessment, Chinese Research Academy of Environmental Sciences, Beijing, 100012, P.R. China. E-mail: jiangxia@craes.org.cn; Fax: +86 10 84913869; Tel: +86 10 84913869
bDepartment of Environmental Science and Engineering, Fudan University, Shanghai 200433, P.R. China
First published on 15th September 2015
Abstract
The sorption of phosphorus under different ionic strengths was investigated in four types of sediment samples from the Chinese Dianchi Lake by kinetic and batch equilibrium experiments. The results showed that the sorption rate followed both the pseudo-first order and pseudo-second order. However, the pseudo-second order could better describe the sorption kinetics than the pseudo-first order. Sediments 1 (S1) and 4 (S4) had higher sorption capacities than Sediments 2 (S2) and 3 (S3). The sorption capacities of the four sediments were highly influenced by ionic strength, and low ionic strength was favorable for P uptake. The sorption kinetics of P to sediment was regulated by the surface-diffusion mechanism, and the diffusion rate of P from the liquid–sediment boundary to sediment surface determined its sorption rate. The classic Langmuir isotherm equation was modified to describe P sorption on sediments, and the modified Langmuir isotherm could describe the P sorption well. The native adsorbed phosphorus (NAP), equilibrium phosphorus concentration (EPC0), and maximum phosphorus capacity (Qm) varied with ionic strength. The Fe/Al oxide in the sediment had a significant relationship (R2 > 0.95, P < 0.05) with NAP, EPC0, and Qm. The positions of S1 and S4 had the potential risk of P release, whereas S2 and S3 play a pool role of P.
1. Introduction
Phosphorus, as one of the main nutrients, is found in soils, sediments, water, and organisms.1,2 However, excessive P can cause eutrophication and the chaotic growth of undesirable algae and other aquatic plants,3,4 which has become a widespread environmental problem. P in sediment exists in different forms (e.g., calcium, iron, or aluminum complex salts and organic species), can be adsorbed onto the surface of minerals, and is an important part of many structural and biochemically functional components for cell growth of plants.5,6 Lake sediments can act as either P sources or pools to the overlying water column. Therefore, understanding the transformation process of P at the sediment–water interface is increasingly becoming important.In the past, P sorption characteristics on homogeneous sorbents such as goethite, which contain pure minerals,7,8 has been described by different models that have been used to describe P sorption on sediments. For instance, simple Langmuir and Freundlich models were extensively used to describe the sorption features of P on sediments.9,10 However, the sediment, which is different from homogeneous sorbents, has complex components. P exists in the sediment in various forms, such as Ca–P, Al–P, Fe–P, and organic P. The native adsorbed phosphorus (NAP) can immediately participate in the sorption or desorption process to influence the sorption or desorption capacity of the sediment. Researchers used oxalate-extractable phosphorus to represent NAP in sediments.11,12 However, NAP content may change with sediment composition or environmental conditions such as lake chemistry.6,13 Therefore, NAP cannot be neglected or replaced by oxalate-extractable P in the descriptive model for sorption or desorption processes.
Many experimental conditions that can influence the sorption process, such as pH and temperature, have been investigated. A previous study14 indicated that P sorption increased as the pH increased from pH 2 to pH 4 in Taihu Lake sediments (China). Detenbeck and Brezonik13 found that the sorption decreased when pH increased from pH 4 to pH 6 in Little Rock Lake (Wisconsin, USA). Furthermore, some studies indicated that P sorption increased with temperature increasing, thus decreasing the soluble P fraction.15,16 These studies indicated that pH and temperature effects differ by sediment. The ionic strength effect has been explored in the area of organic matter (OM) sorption. Some researchers found that under high ionic strength conditions, the water solubility of OM adhered to soil particles decreased because of the salting-out effect.17 Moreover, the abundant cation in the liquid–solid system could interact with the negative charges of the solid surface. The abundant anion would compete on the active site during the anion sorption process. However, few studies focused on the effect of ionic strength on P sorption in the sediments. Research into P sorption has also been conducted in diverse sediment types. These studies have almost exclusively focused on sediments in shallow lakes, estuaries, or reservoirs,18–20 whereas information about P sorption or desorption of sediments in plateau lakes are limited or nonexistent. Therefore, the effect of ionic strength on P in sediments should be emphasized. Dianchi Lake, a typical plateau lake, is located in southwestern China and was selected as the research area.
The objectives of the present study are mainly to describe the sorption kinetics of P onto sediments from Dianchi Lake and to model P sorption processes by modified isotherm. Meanwhile, the effect of ionic strength is evaluated. This study provides the useful information for sediment management.
2. Materials and methods
2.1 Area description
Dianchi Lake is a plateau lake (water surface elevation = 1886 m) located in southwestern China (24°48′N; 102°40′E). The lake covers an area of 330 km2 with a volume of approximately 1.2 × 1011 m3. The lake is divided into two sections. The northwestern part is called Caohai and the southern part is called Waihai.21,22 These two sections are separated by Hubing Road (Fig. 1) with a sluice. This study area is located beside Hubing Road in Waihai and encloses two large enclosures (Fig. 1).2.2 Sediment collection and characterization
Four composite surface sediment samples (each N = 7) were collected at the sites shown in Fig. 1 in April 2014. Sediment 1 (S1) was collected in a small enclosure and Sediment 2 (S2) was collected at the border of the enclosure. Sediments 3 (S3) and 4 (S4) were located between two large enclosures and in the deepest area, respectively. The sediment samples were immediately brought to the laboratory, where they were freeze-dried, ground, and sifted through 100 mesh (0.150 mm) sieve to obtain uniform size.23 The total phosphorus (TP) of the sediments was measured by using the standard measurement and test (SMT).24 In this procedure, HCl (3.5 mol L−1) was used as extractants and an intake of 200 mg of sediment which was calcined at 450 °C for 3 hours was necessary for the extraction. Oxalate-extractable P (Ox-P) in sediment was measured using ammonium chloride (NH4Cl) as extractants.25 The P concentration in the solution was determined by the molybdenum blue method after the solution was filtered (0.45 μm filtration membrane).26 The pH of the sediment was measured in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 (w/v) mixture of sediment with deionized water or 0.01 mol L−1 KCl solution.27 OM content was calculated according to the loss on ignition to constant mass (4 h) at 550 °C. The specific surface area was measured by the N2 adsorption method with the surface analyzer (Micromeritics, USA) in the relative atmospheric pressure range of 0.001 to 0.995. The chemical composition of the samples was determined by X-ray fluorescence analyzer (S4 EXPLORER, Germany). The main properties of the collected sediments are shown in Table 1.
2.5 (w/v) mixture of sediment with deionized water or 0.01 mol L−1 KCl solution.27 OM content was calculated according to the loss on ignition to constant mass (4 h) at 550 °C. The specific surface area was measured by the N2 adsorption method with the surface analyzer (Micromeritics, USA) in the relative atmospheric pressure range of 0.001 to 0.995. The chemical composition of the samples was determined by X-ray fluorescence analyzer (S4 EXPLORER, Germany). The main properties of the collected sediments are shown in Table 1.
| Propert (unit) | S1 | S2 | S3 | S4 |
|---|---|---|---|---|
| TP (mg kg−1) | 1395.98 ± 60.48 | 1150.59 ± 58.45 | 996.87 ± 50.87 | 950.61 ± 55.89 |
| TN (mg kg−1) | 2572.59 ± 75.69 | 3127.40 ± 73.38 | 3312.34 ± 69.35 | 1595.07 ± 54.84 |
| Ox-P (mg kg−1) | 323.21 ± 26.21 | 265.33 ± 20.05 | 287.56 ± 21.45 | 345.12 ± 28.21 |
| OM (%) | 13.75 ± 0.24 | 10.88 ± 0.18 | 9.68 ± 0.16 | 12.89 ± 0.21 |
| pH [H2O] | 7.39 ± 0.07 | 7.93 ± 0.06 | 7.88 ± 0.10 | 7.28 ± 0.08 |
| pH [KCl] | 7.24 ± 0.06 | 7.79 ± 0.11 | 7.75 ± 0.08 | 7.11 ± 0.05 |
| Fe oxide (%) | 8.70 ± 0.07 | 6.56 ± 0.06 | 8.78 ± 0.09 | 8.43 ± 0.05 |
| Ca oxide (%) | 26.2 ± 0.4 | 15.9 ± 0.3 | 22.5 ± 0.4 | 26.5 ± 0.3 |
| Al oxide (%) | 16.6 ± 0.3 | 13.1 ± 0.3 | 11.8 ± 0.2 | 16.6 ± 0.2 |
| Mg oxide (%) | 2.14 ± 0.04 | 1.31 ± 0.03 | 1.5 ± 0.03 | 2.14 ± 0.02 |
| BET surface area (m2 g−1) | 10.44 ± 0.31 | 7.70 ± 0.28 | 4.56 ± 0.19 | 9.12 ± 0.25 |
2.3 Sorption kinetics study
P sorption kinetics was examined at 4 different ionic strengths of 0, 0.001, 0.01, and 0.1 M KCl. The stock solution, which was obtained by dissolving the salt KH2PO4 and KCl in deionized water, was prepared within 24 h before use and was stored in the dark at 4 °C until use. Four different sediments (1 g) were added to a series of 150 mL conical flasks containing 50 mL of 10 mg L−1 P solution with different ionic strengths (viz. 0, 0.001, 0.01, and 0.1 M KCl). The sediment suspensions were immediately covered with parafilm and constantly agitated on a temperature-controlled shaker at 200 rpm under a constant temperature of 25 ± 2 °C. At 11 different time intervals (0.5, 1, 2, 4, 8, 12, 24, 36, 48, 60, and 72 h), the suspensions were taken from each flask, centrifuged, filtered (0.45 μm), and analyzed for P content. The experiments were conducted in triplicate, and the sediment sample (1 g) to solution (50 mL) ratio was assumed constant throughout the incubation period.2.4 Sorption isotherm tests
The sorption isotherms of P by sediments were obtained by using batch experiments. The sediment samples of 1 g in triplicate were added to a series of 150 mL conical flasks containing 50 mL P solution at different initial P concentrations (0, 1, 5, 10, 15, and 20 mg L−1 as KH2PO4). In this sorption isotherm tests, four different ionic strengths were studied by using 0, 0.001, 0.01, and 0.1 M KCl. The conical flasks were agitated on a temperature-controlled shaker at 200 rpm under a constant temperature of 25 ± 2 °C for 48 h equilibration period. The suspensions were centrifuged, filtered (0.45 μm), and analyzed for P.2.5 Kinetic and equilibrium sorption data fitting
The P uptake amount onto the different sediments at each time, Qt (mg g−1), was calculated by using a mass balance relationship:
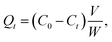 | (1) |
To investigate the mechanism of sorption and potential rate-controlling steps, pseudo-first and -second order models and Weber–Morris model were used to describe the sorption kinetics.28,29
The pseudo-first order model is expressed as follows:
| Qt = Qe(1 − e−K1t), | (2) |
The pseudo-second order model can be expressed as follows:
 | (3) |
The Weber–Morris model is expressed as follows:
| Qt = Kat0.5 + C, | (4) |
Sorption isotherm data are also fitted by using Langmuir and Freundlich sorption models.
The Langmuir model is expressed as follows:
 | (5) |
The Freundlich model is expressed as follows:
| Qe = KfCen, | (6) |
When the sediment interacts with water, P will be exchanged between the water and sediment during the interface until a dynamic equilibrium is reached. The Langmuir sorption isotherm usually describes the equilibrium situation.6 Further studies indicated that the Langmuir model has been used widely to describe the anion sorption by soil and sediments10,32 despite their heterogeneous nature. However, the Langmuir model could not work perfectly because of NAP in the sediment.32 Therefore, to study P sorption directly, eqn (5) is unreasonable because the NAP in the sediment is also included in the sorption equilibrium.33 By considering NAP, the equilibrium conditions of sorption trials could be described as follows:
 | (7) |
 | (8) |
Thereafter, the modified Langmuir sorption isotherm can be expressed as follows:
 | (9) |
P sorption properties on lake sediments that contain different amounts of NAP could be described by eqn (9). By using this model, the data of sorption isotherm trials could be nonlinearly fitted properly with the least square method. The value of Qm and K were obtained. Thereafter, NAP could be calculated by eqn (8). The zero equilibrium phosphorus concentration (EPC0) is the concentration in which no net sorption or desorption of P occurs, and the original sediment and water P concentrations are in dynamic equilibrium. When Qe = 0 or Cadd = Ce, the EPC0 value (Ce) according to eqn (9) is expressed as follows:34
 | (10) |
The newly added P is assumed to directly compete with NAP and they have the same values of Qm and K. Kp can be calculated as follows:
 | (11) |
3. Results and discussion
3.1 P sorption kinetics
The sorption kinetic was most commonly described with the pseudo-first and pseudo-second order models.35 Three steps are considered in kinetic models: (1) the sorbate ions diffuse from the liquid phase to the liquid–solid boundary; (2) the sorbate ions move from the liquid–solid boundary to the solid surface; (3) the sorbate ions diffuse into the particles.36 The experimental results corresponding to the P sorption onto four sediments under study (Qt versus t) together with fitting to the kinetic equations are shown in Fig. 2 and Table 2. The kinetic behavior of P sorption onto these four sediments was examined by 72 h contact time. In these figures, the P sorption from solution onto four sediments was relatively rapid in the first few hours (0 h to 8 h) and the sorption rate progressively diminished until an apparent equilibrium (8 h to 48 h) was reached. This result was similar to early published results on P sorption onto sediments.37,38 In the first few hours, the active sorption sites were occupied rapidly. In the next few hours, the active sorption sites were reduced and the P sorption rate decreased. After 48 h, the sorption equilibrium could be achieved or desorption might occur for all sediments.| Sample | Ionic strength (M) | Pseudo-first order model | Pseudo-second order model | Weber–Morris model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qe (mg g−1) | K1 (h−1) | R2 | Qe (mg g−1) | K2 (g mg−1 h−1) | R2 | C (mg g−1) | Ka (mg g−1 h−0.5) | R2 | ||
| S1 | 0 | 0.396 ± 0.009 | 2.161 ± 0.350 | 0.9487 | 0.409 ± 0.005 | 9.681 ± 1.267 | 0.9881 | 0.309 ± 0.018 | 0.028 ± 0.007 | 0.8329 |
| 0.001 | 0.406 ± 0.008 | 2.225 ± 0.338 | 0.9556 | 0.418 ± 0.004 | 10.095 ± 1.187 | 0.9908 | 0.324 ± 0.021 | 0.027 ± 0.008 | 0.7572 | |
| 0.01 | 0.402 ± 0.008 | 2.517 ± 0.388 | 0.9596 | 0.414 ± 0.004 | 11.915 ± 1.490 | 0.9911 | 0.324 ± 0.016 | 0.026 ± 0.006 | 0.8409 | |
| 0.1 | 0.330 ± 0.007 | 2.597 ± 0.438 | 0.9534 | 0.340 ± 0.004 | 14.973 ± 2.262 | 0.9874 | 0.263 ± 0.013 | 0.022 ± 0.005 | 0.8596 | |
| S2 | 0 | 0.299 ± 0.007 | 1.722 ± 0.256 | 0.9500 | 0.310 ± 0.003 | 9.583 ± 0.970 | 0.9912 | 0.222 ± 0.009 | 0.024 ± 0.004 | 0.9319 |
| 0.001 | 0.302 ± 0.007 | 1.576 ± 0.230 | 0.9498 | 0.313 ± 0.003 | 8.624 ± 0.827 | 0.9916 | 0.218 ± 0.010 | 0.026 ± 0.004 | 0.9351 | |
| 0.01 | 0.295 ± 0.006 | 1.881 ± 0.269 | 0.9556 | 0.305 ± 0.003 | 11.221 ± 1.150 | 0.9918 | 0.226 ± 0.008 | 0.022 ± 0.003 | 0.9504 | |
| 0.1 | 0.226 ± 0.005 | 1.314 ± 0.187 | 0.9496 | 0.235 ± 0.003 | 9.224 ± 0.951 | 0.9893 | 0.167 ± 0.008 | 0.016 ± 0.003 | 0.9083 | |
| S3 | 0 | 0.366 ± 0.009 | 1.950 ± 0.339 | 0.9366 | 0.379 ± 0.005 | 9.124 ± 1.348 | 0.9831 | 0.260 ± 0.018 | 0.035 ± 0.007 | 0.8934 |
| 0.001 | 0.372 ± 0.009 | 2.130 ± 0.384 | 0.9364 | 0.385 ± 0.006 | 9.708 ± 1.545 | 0.9815 | 0.270 ± 0.017 | 0.032 ± 0.006 | 0.8872 | |
| 0.01 | 0.373 ± 0.009 | 1.999 ± 0.340 | 0.9401 | 0.386 ± 0.005 | 9.186 ± 1.291 | 0.9850 | 0.283 ± 0.015 | 0.028 ± 0.006 | 0.8804 | |
| 0.1 | 0.300 ± 0.008 | 2.097 ± 0.406 | 0.9259 | 0.311 ± 0.005 | 12.020 ± 2.185 | 0.9760 | 0.226 ± 0.020 | 0.024 ± 0.007 | 0.7362 | |
| S4 | 0 | 0.429 ± 0.008 | 2.594 ± 0.370 | 0.9663 | 0.440 ± 0.004 | 11.846 ± 1.389 | 0.9926 | 0.352 ± 0.01 | 0.024 ± 0.004 | 0.9209 |
| 0.001 | 0.425 ± 0.008 | 2.817 ± 0.450 | 0.9619 | 0.437 ± 0.005 | 13.294 ± 2.074 | 0.9883 | 0.361 ± 0.004 | 0.018 ± 0.002 | 0.9752 | |
| 0.01 | 0.421 ± 0.007 | 2.589 ± 0.357 | 0.9684 | 0.432 ± 0.004 | 12.211 ± 1.385 | 0.9931 | 0.354 ± 0.010 | 0.020 ± 0.004 | 0.8979 | |
| 0.1 | 0.381 ± 0.007 | 3.312 ± 0.585 | 0.9634 | 0.390 ± 0.005 | 18.721 ± 3.744 | 0.9854 | 0.330 ± 0.004 | 0.013 ± 0.001 | 0.9666 | |
The rate constants and parameters of the pseudo-first and -second order models derived from the nonlinear regression (eqn (2) and (3)) are shown in Table 2. The kinetic sorption data of P by sediments were well fitted with the pseudo-second order model compared with the pseudo-first order model, as indicated by higher correlation coefficients (R2 > 0.97). S1 and S4 had higher phosphorus uptake amounts at the equilibrium (Qe) than S2 and S3 (Table 2). This result could be attributed to the relatively high Fe, Al, and Ca oxide contents among sediments to provide abundant specific areas (Table 1) for P sorption.12 Regarding these four sediments, they displayed different P sorption kinetic constants, and the K2 values were S4 > S1 > S3 > S2 in the same ionic strength. The comparison of the kinetic constants obtained for sediments with the physicochemical characterization (Table 1) showed that S1 and S4 had relatively high OM, Fe, Al, and Ca oxides. A previous study39 indicated that sorption velocity was favored by the OM content of the samples. Therefore, S1 and S4 displayed not only the largest Qe but also the largest K2 and Fe. Al and Ca oxides favored both the uptake amount and velocity of P sorption; this result is consistent with literature.40,41 Regarding the pH measured in either in H2O or in KCl, the P uptake amount and K2 values were enhanced by relatively low pH values because the increase in pH might prevent P sorption onto Fe, Al, and Ca oxides according to the speciation shifting of phosphate from H2PO4− to HPO42−; the surface charges of Fe, Al, and Ca oxides were also modified.42,43 The kinetic sorption data showed that P sorption onto S1 and S4 occurred rapidly compared with S2 and S3.
However, the diffusion process of P in pores or that from the liquid–sediment boundary to sediment surfaces was inconclusive. To reveal the relative contribution of surface and intra-particle diffusion to the entire sorption process, the kinetic sorption data of P in the sediment were further fitted with the Weber–Morris model. The sorption process could be divided into three steps: a sharp rise portion, a less-sharp rise portion, and a plateau (Fig. 3). In the first step, approximately 40.0% to 73.1% of P was sorbed into the sediment because of the occupation of exterior activated site by various physicochemical interactions, such as covalent forces, hydrophobic interaction, and van der Waals forces. Furthermore, the thickness of the boundary layer (C) for S1 and S4 (Table 2) was relatively larger compared with S2 and S3.44 The surface sorption played a significant role for P in the sediment. In the second step, approximately 16.4% to 19.6% of the P that sorbed into the sediment was slowly diffused from the liquid–sediment boundary to the sediment surfaces. In the third step, the P sorption approached equilibrium and desorption processes might occur. By contrast, if the regression of Qt against t1/2 was linear and the regression line passed through the origin, then the intra-particle diffusion was presumed to be the rate-controlling step.36 The Weber–Morris models results in Table 2 and Fig. 3 revealed that Qt was linearly correlated with t1/2 and that all regression lines did not pass through the origin with positive intercepts, thus suggesting that the diffusion on the pores might not be a rate-controlling step for the kinetic sorption of P to sediment. Therefore, the sorption kinetic of P to sediment was regulated by the surface-diffusion mechanism and the diffusion rate of phosphorus from the liquid–sediment boundary to the sediment surface determined their sorption rate.
3.2 Ionic strength effects on P sorption
The effect of ionic strength (with KCl as background electrolyte) on P sorption by four sediments was investigated by using batch methods. P uptake amount was statistically insignificant in the 0, 0.001, and 0.01 M KCl treatments and in the 0.001 M KCl solution (Fig. 2); the sediment had relatively high sorption capacity. When the ionic strength increased from 0 M KCl to 0.01 M KCl, the introduction of cations (K+) would make the plane potential less negative to increase the electrostatic potential (ψ), thereby facilitating P uptake capacity.45 Although chloride ions with low ionic strengths (0, 0.001, and 0.01 M) would compete with P ions on the active sites, the high values of the water-soluble P and ion concentrations in the solution might not cause a noticeable variation in P uptake amount. As the ionic strength increased from 0.01 M KCl to 0.1 M KCl, the P uptake amount considerably decreased. Previous studies46,47 suggested that the ion exchange reaction was the mechanism for adverse anion effects on P sorption. The introduction of competing anions would reduce the uptake amount of P. The chloride ions would compete with phosphate ions on the active site to replace the pre-adsorbed phosphate.3.3 Equilibrium sorption of P
The effects of ionic strength on the equilibrium sorption isotherm of P were studied, and Fig. 4 shows the results. The P uptake amounts were S4 > S1 > S3 > S2 in 0, 0.001, 0.01, and 0.1 M ionic strength. P uptake amount was statistically insignificant in the 0, 0.001, and 0.01 M KCl treatments, and in the 0.1 M KCl solution, the P uptake amount was relatively low. The P uptake amount also increased with increasing P initial concentration, and the increase rate decreased as the equilibrium concentration increased in 0, 0.001, and 0.01 M KCl treatments (Fig. 4a–c). However, when the ionic strength increased to 0.1 M, the increased rate of P uptake amount became unstable (Fig. 4d). This result might be explained on the basis of the ion exchange mechanism that played an important role in the sorption process, and the chloride ions would compete with phosphate ions on the active sites.The fitting of sorption isotherm equations to experimental sorption is usually a significant aspect of understand sorption behavior. In this study, the sorption isotherms of P by four sediments are presented in Fig. 4, and the isotherm data were fitted with the modified Langmuir and Freundlich models (Table 3). As evidenced by the correlation coefficient R2, the P sorption isotherm of four sediments can be described by using the modified Langmuir model (R2 > 0.9885) rather than using the Freundlich model (R2 < 0.9806). The Langmuir sorption isotherm provided a good estimate of the theoretical sorption maxima (Qm), thus reflecting the sorption capacity of sorbent and the affinity parameter K. The values of Qm and K in this study were obtained in Table 3, and the Langmuir sorption maxima and the affinity parameter of these four sediments ranged from 0.527 mg g−1 to 1.126 mg g−1 and 0.252 mg g−1 to 0.983 L mg−1 in different ionic strengths, respectively. The change in Qm and K was statistically insignificant to the change in ionic strength from 0 M to 0.01 M because the introduction of cations (K+) would increase the electrostatic potential (ψ) to facilitate the P uptake capacity or a small amount of chloride ions would compete with phosphate ions on the active site to decrease the P uptake amount. However, when the KCl concentration increased from 0.01 M to 0.1 M, the ionic strength reached a certain level in which the background electrolyte (KCl) not only reduced the uptake amount but also reduced the binding affinity of the absorbent. The introduction of chloride ions would change the sorption atmosphere of the sorbent surface. Therefore, when the KCl concentration increased to 0.1 M, chloride ions competed on the active sites with phosphate ions, and the atmosphere of the sorbent surface could be modified.
| Sediment sample | Ionic strength (M) | C0e (mg L−1) | Q0e (mg g−1) | Langmuir: | Freundlich: Qe = KfCen | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fitting results | Calculated results | Fitting results | ||||||||||
| K (L mg−1) | Qm (mg g−1) | R2 | EPC0 (mg L−1) | NAP (mg g−1) | Kp (L g−1) | Kf (L g−1) | n | R2 | ||||
| #1 | 0 | 0.072 | 0.0036 | 0.734 | 0.970 | 0.9993 | 0.065 | 0.052 | 0.800 | 0.307 | 0.543 | 0.9413 |
| 0.001 | 0.061 | 0.0031 | 0.606 | 1.056 | 0.9998 | 0.069 | 0.041 | 0.597 | 0.307 | 0.573 | 0.9611 | |
| 0.01 | 0.072 | 0.0036 | 0.669 | 0.980 | 0.9984 | 0.068 | 0.049 | 0.718 | 0.304 | 0.548 | 0.9559 | |
| 0.1 | 0.146 | 0.0073 | 0.562 | 0.923 | 0.9931 | 0.116 | 0.077 | 0.665 | 0.269 | 0.552 | 0.9386 | |
| #2 | 0 | 0.093 | 0.0047 | 0.380 | 0.604 | 0.9949 | 0.039 | 0.025 | 0.657 | 0.146 | 0.512 | 0.9567 |
| 0.001 | 0.072 | 0.0036 | 0.456 | 0.650 | 0.9894 | 0.033 | 0.024 | 0.729 | 0.132 | 0.546 | 0.9806 | |
| 0.01 | 0.061 | 0.0031 | 0.348 | 0.591 | 0.9971 | 0.026 | 0.015 | 0.605 | 0.148 | 0.492 | 0.9592 | |
| 0.1 | 0.188 | 0.0094 | 0.252 | 0.527 | 0.9911 | 0.065 | 0.033 | 0.510 | 0.107 | 0.548 | 0.9458 | |
| #3 | 0 | 0.072 | 0.0036 | 0.594 | 0.742 | 0.9957 | 0.041 | 0.034 | 0.839 | 0.219 | 0.503 | 0.9566 |
| 0.001 | 0.051 | 0.0025 | 0.701 | 0.718 | 0.9885 | 0.027 | 0.027 | 1.015 | 0.240 | 0.457 | 0.9248 | |
| 0.01 | 0.083 | 0.0041 | 0.617 | 0.734 | 0.9951 | 0.045 | 0.040 | 0.889 | 0.221 | 0.485 | 0.9387 | |
| 0.1 | 0.157 | 0.0078 | 0.438 | 0.702 | 0.9956 | 0.078 | 0.053 | 0.675 | 0.150 | 0.604 | 0.9611 | |
| #4 | 0 | 0.061 | 0.0031 | 0.802 | 1.091 | 0.9992 | 0.070 | 0.054 | 0.774 | 0.372 | 0.565 | 0.9492 |
| 0.001 | 0.051 | 0.0025 | 0.983 | 1.053 | 0.9940 | 0.054 | 0.053 | 0.983 | 0.395 | 0.543 | 0.9344 | |
| 0.01 | 0.051 | 0.0025 | 0.632 | 1.126 | 0.9991 | 0.065 | 0.038 | 0.581 | 0.358 | 0.577 | 0.9677 | |
| 0.1 | 0.104 | 0.0052 | 0.637 | 1.025 | 0.9902 | 0.103 | 0.069 | 0.666 | 0.280 | 0.585 | 0.9317 | |
Theoretically, EPC0 and NAP have the same values for the same sediment. Some studies12,48 and even recent research11 on the effect of different conditions on P sorption to sediments used oxalate-extractable P to represent NAP in sediments. However, in the current study, the same sediment had different EPC0 and NAP values (Table 3) in different ionic strengths, and the NAP values varied greatly with changing ionic strength, thus indicating that the NAP value would vary with experimental conditions, such as ionic strength, temperature, and pH. Furthermore, the oxalate-extractable P could not represent NAP. The sorption behavior of the same sediment was studied in different experimental conditions. The NAP of different sediments in different ionic strengths was obtained. S1 and S4 had relatively higher NAP (0.038 mg g−1 to 0.077 mg g−1) compared with S2 and S3 (0.015 mg g−1 to 0.053 mg g−1) (Table 3). For the same sediment in Table 3, as ionic strength ranging from 0 M to 0.01 M, NAP had a slight change. When the ionic strength increased to 0.1 M, the NAP reached a high value. In addition, the Kp value reflects the relative affinities of the solid phase for P. The large value of Kp indicated the high ability of P sorption. Table 3 indicated that S1 and S4 have the better P sorption capacity than S2 and S3 according to Kp value. Researchers have concluded that sediment composition significantly influenced NAP, EPC0, and Qm values. The inter-correlations among NAP, EPC0, Qm, Ca oxide, and Fe/Al oxide are shown in Table 4. The Fe/Al oxides in sediment have significant relationships (R2 > 0.95, P < 0.05) with NAP, EPC0, and Qm. The relationship between NAP and Ca oxide was also significant. This relation demonstrated that Fe/Al and Ca oxides played major roles in P sorption. The metal hydroxides of Fe, Al, and Ca are widely known to be the main absorbents for P. Therefore, the high content of Fe, Al, and Ca oxides in S1 and S4 caused the high P sorption capacity.
| NAP | EPC0 | Qm | Fe/Al oxide | Ca oxide | |
|---|---|---|---|---|---|
| a *P < 0.05. | |||||
| NAP | 1 | ||||
| EPC0 | 0.975* | 1 | |||
| Qm | 0.986* | 0.973* | 1 | ||
| Fe/Al oxide | 0.986* | 0.983* | 0.956* | 1 | |
| Ca oxide | 0.951* | 0.860 | 0.933 | 0.899 | 1 |
3.4 The dual nature of sediments as a pool and source of P in the study area
Depending on the dynamic equilibrium between solution and solid phases, P can either be adsorbed or desorbed from sediments. The determination of sediment EPC0 value can reveal the direction of soluble reactive phosphorus (SRP) flux.49 If EPC0 is greater than the SRP concentration in pore water, then the sediment will release P and vice versa. In the present study, the sorption isotherms produce EPC0. The SRP concentrations in pore water (Cpwp) and overlying water (Cowp) are shown in Table 5. When Cpwp = EPC0, P sorbed = 0 (neither pool nor source); when Cpwp > EPC0, P sorbed > 0 (sediment serves as pool of phosphorus); when Cpwp < EPC0, P sorbed < 0 (sediment serves as source of phosphorus).6 For S1 and S4, the values of Cpwp were smaller than EPC0, and these two sediments were sources of P. Furthermore, in the study area, the ionic strength in overlying water ranges from 10−3 mg L−1 to 10−2 mg L−1, and the average value of sediment cation exchange capacity (CEC) is approximately 0.25 mol (+) kg−1. The differences of ionic strength may cause the change of EPC0, which is one of the main reasons for sediment serving as source or pool of phosphorus. Therefore, this finding explained why SRP concentrations in the overlying water (Cowp) of these two points were higher than the other two points. For S2 and S3, the values of Cpwp were larger than EPC0, and these two sediments have a pool role of P, thus resulting in lower Cowp than that in S1 and S4.| Sediment sample | EPC0 (mg L−1) | Cpwp (mg L−1) | Cowp (mg L−1) |
|---|---|---|---|
| #1 | 0.065 ± 0.013 | 0.032 ± 0.009 | 0.022 ± 0.002 |
| #2 | 0.039 ± 0.014 | 0.053 ± 0.011 | 0.015 ± 0.003 |
| #3 | 0.041 ± 0.016 | 0.049 ± 0.012 | 0.017 ± 0.002 |
| #4 | 0.070 ± 0.014 | 0.015 ± 0.008 | 0.029 ± 0.004 |
4. Conclusion
The study of P sorption kinetics is a valuable tool for evaluating the system response to P external loadings. The sorption rate followed both the pseudo-first order and pseudo-second order. The results showed that the pseudo-second order could better describe the sorption kinetics than the pseudo-first order. The sorption capacities of four sediments were S4 > S1 > S3 > S2 and was highly influenced by ionic strength. Low ionic strength was favorable for P uptake. By contrast, the sorption kinetic of P to sediment was regulated by the surface-diffusion mechanism, and the diffusion rate of P from the liquid–sediment boundary to the sediment surface determines their sorption rate. The modified Langmuir isotherm equation interprets well the P sorption data of these four sediments. The Fe/Al oxide in sediment has a significant relationship (R2 > 0.95, P < 0.05) with NAP, EPC0, and Qm. Comparing the EPC0 and Cpwp values, S1 and S4 are P sources, whereas S2 and S3 have pool role, providing useful information for sediment management.Acknowledgements
This study was supported by the Major Science and Technology Program for Water Pollution Control and Treatment (No. 2012ZX07102-004).References
- X. L. Huang and J. Z. Zhang, Chemosphere, 2011, 85, 1227–1232 CrossRef CAS PubMed.
- V. Baldy, M. Trémolières, M. Andrieu and J. Belliard, Hydrobiologia, 2006, 575, 343–351 CrossRef.
- L. E. de-Bashan and Y. Bashan, Water Res., 2004, 38, 4222–4246 CrossRef CAS PubMed.
- C. Yan, L. Zhang, X. Luo and Z. Zheng, Ecol. Eng., 2013, 51, 24–32 CrossRef PubMed.
- R. A. Dorich, D. W. Nelson and L. E. Sommers, Agric., Ecosyst. Environ., 1984, 11, 253–264 CrossRef CAS.
- A. Zhou, H. Tang and D. Wang, Water Res., 2005, 39, 1245–1254 CrossRef CAS PubMed.
- T. Hiemstra and W. H. van Riemsdijk, J. Colloid Interface Sci., 1996, 179, 488–508 CrossRef CAS.
- J. S. Geelhoed, T. Hiemstra and W. H. van Riemsdijk, Environ. Sci. Technol., 1998, 32, 2119–2123 CrossRef CAS.
- P. Lopez, X. Lluch, M. Vidal and J. A. Morgui, Estuarine, Coastal Shelf Sci., 1996, 42, 185–196 CrossRef CAS.
- M. Del Bubba, C. A. Arias and H. Brix, Water Res., 2003, 37, 3390–3400 CrossRef CAS.
- L. Huang, L. Fu, C. Jin, G. Gielen, X. Lin, H. Wang and Y. Zhang, Ecol. Eng., 2011, 37, 1515–1522 CrossRef PubMed.
- D. Y. F. Lai and K. C. Lam, Ecol. Eng., 2009, 35, 735–743 CrossRef PubMed.
- N. E. Detenbeck and P. L. Brezonik, Environ. Sci. Technol., 1991, 25, 395–403 CrossRef CAS.
- S. R. Wang, X. C. Jin, Y. Pang, H. C. Zhao and X. N. Zhou, J. Colloid Interface Sci., 2005, 285, 448–457 CrossRef CAS PubMed.
- R. N. Sah and D. S. Mikkelsen, Plant Soil, 1986, 95, 173–181 CrossRef CAS.
- N. J. Barrow, J. Soil Sci., 1979, 30, 271–297 CrossRef CAS PubMed.
- A. Turner and M. C. Rawling, Water Res., 2001, 35, 4379–4389 CrossRef CAS.
- S. Leston, A. I. Lillebo and M. A. Pardal, Estuarine, Coastal Shelf Sci., 2008, 77, 688–696 CrossRef PubMed.
- X. Y. Wang, L. P. Zhang, H. Zhang, X. Y. Wu and D. L. Mei, Environ. Earth Sci., 2012, 67, 15–22 CrossRef CAS.
- Q. R. Wang and Y. C. Li, J. Soils Sediments, 2010, 10, 1159–1173 CrossRef CAS.
- X. Qu, L. Xie, Y. Lin, Y. Bai, Y. Zhu, F. Xie, J. P. Giesy and F. Wu, Environ. Sci. Pollut. Res., 2013, 20, 7413–7423 CrossRef CAS PubMed.
- C. Huang, X. Wang, H. Yang, Y. Li, Y. Wang, X. Chen and L. Xu, Sci. Total Environ., 2014, 485–486, 1–11 CrossRef CAS PubMed.
- X. Jin, S. Wang, Y. Pang, H. Zhao and X. Zhou, Colloids Surf., A, 2005, 254, 241–248 CrossRef CAS PubMed.
- V. Ruban, J. F. Lopez-Sanchez, P. Pardo, G. Rauret, H. Muntau and P. Quevauviller, J. Environ. Monit., 2001, 3, 121–125 RSC.
- L. Huang, L. Li, L. Huang, G. Gielen, Y. Zhang and H. Wang, J. Soils Sediments, 2011, 12, 443–455 CrossRef.
- J. Murphy and J. P. Riley, Anal. Chim. Acta, 1962, 27, 31–36 CrossRef CAS.
- X. Liao, C. Zhang, L. Yao, J. Li, M. Liu, L. Xu and M. Evalde, Sci. Total Environ., 2014, 473–474, 530–536 CrossRef CAS PubMed.
- Y. S. Ho, D. A. J. Wase and C. F. Forster, Environ. Technol., 1996, 17, 71–77 CrossRef CAS PubMed.
- Y. S. Ho and G. McKay, Process Biochem., 1999, 34, 451–465 CrossRef CAS.
- W. J. Weber and J. C. Morris, ASCE J. Sanit. Eng. Div., 1963, 89, 31–59 Search PubMed.
- C. Sairam Sundaram, N. Viswanathan and S. Meenakshi, Bioresour. Technol., 2008, 99, 8226–8230 CrossRef CAS PubMed.
- W. A. House and F. H. Denison, Water Res., 2000, 34, 1187–1200 CrossRef CAS.
- A. Aminot and F. Andrieux, Water Res., 1996, 30, 2805–2811 CrossRef CAS.
- X. Wang, L. Zhang, H. Zhang, X. Wu and D. Mei, Environ. Earth Sci., 2011, 67, 15–22 CrossRef.
- P. Janoš, P. Michálek and L. Turek, Dyes Pigm., 2007, 74, 363–370 CrossRef PubMed.
- X. Wang, L. Shu, Y. Wang, B. Xu, Y. Bai, S. Tao and B. Xing, Environ. Sci. Technol., 2011, 45, 9276–9283 CrossRef CAS PubMed.
- M. Otero, J. P. Coelho, E. T. Rodrigues, M. A. Pardal, E. B. Santos, V. I. Esteves and A. I. Lillebo, Mar. Pollut. Bull., 2013, 77, 361–366 CrossRef CAS PubMed.
- O. Eljamal, J. Okawauchi, K. Hiramatsu and M. Harada, Environ. Earth Sci., 2012, 68, 859–863 CrossRef.
- P. J. A. Howard and D. M. Howard, Biol. Fertil. Soils, 1990, 9, 306–310 CrossRef CAS.
- M. B. McGechan, Biosystems Eng., 2002, 82, 115–130 CrossRef.
- H. K. Pant and K. R. Reddy, J. Environ. Qual., 2001, 30, 1474–1480 CrossRef CAS.
- M. E. Lebo, Mar. Chem., 1991, 34, 225–246 CrossRef CAS.
- J. J. G. Zwolsman, Estuarine, Coastal Shelf Sci., 1994, 39, 227–248 CrossRef CAS.
- X. P. Liao, C. X. Zhang, L. L. Yao, J. L. Li, M. Liu, L. Xu and M. Evalde, Sci. Total Environ., 2014, 473, 530–536 CrossRef PubMed.
- N. S. Bolan, J. K. Syers and R. W. Tillman, J. Soil Sci., 1986, 37, 379–388 CrossRef CAS PubMed.
- L. Huang, W. Qiu, X. Xu and Y. Zhang, Chem. Ecol., 2013, 29, 519–528 CrossRef CAS PubMed.
- J. Liu, L. Wan, L. Zhang and Q. Zhou, J. Colloid Interface Sci., 2011, 364, 490–496 CrossRef CAS PubMed.
- D. Freese, S. Vanderzee and W. H. Vanriemsdijk, J. Soil Sci., 1992, 43, 729–738 CrossRef CAS PubMed.
- W. A. House and F. H. Denison, Environ. Sci. Technol., 2002, 36, 4295–4301 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |





