Inkjet-printed transparent nanowire thin film features for UV photodetectors
Abstract
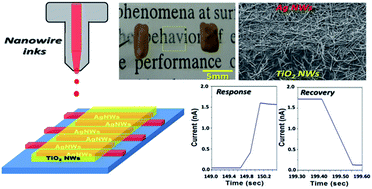
In this study, a simple and effective direct printing method was developed to print patterned nanowire thin films for UV detection. Inks containing silver or titanium dioxide (TiO2) nanowires were first formulated adequately to form stable suspension for inkjet printing applications. Sedimentation tests were also carried out to characterize the terminal velocity and dispersion stability of nanowires to avoid potential nozzle clogging problems. The well-dispersed silver nanowire ink was then inkjet printed on PET films to form patterned electrodes. Above the electrodes, another layer of TiO2 nanowires was also printed to create a highly transparent photodetector with >80% visible transmittance. The printed photodetector showed a fairly low dark current of 10−12–10−14 A with a high on/off ratio of 2000 to UV radiation. Under a bias voltage of 2 V, the detector showed fast responses to UV illumination with a rise time of 0.4 s and a recovery time of 0.1 s. More photo currents can also be collected with a larger printed electrode area. In summary, this study shows the feasibility of applying inkjet printing technology to create nanowire thin films with specific patterns, and can be further employed for photoelectric applications.

 Please wait while we load your content...
Please wait while we load your content...