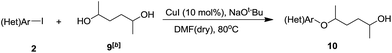
Ligand-free Cu-catalyzed O-arylation of aliphatic diols†
Yufen Zheng,
Wenxing Zou,
Laichun Luo,
Jiabei Chen,
Songwen Lin and
Qi Sun*
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, 38, Xueyuan Road, Haidian Distract, Beijing 100191, PR China. E-mail: sunqi@bjmu.edu.cn
First published on 27th July 2015
Abstract
Coupling reaction between aryl iodides and aliphatic diols was realized with a ligand-free copper catalyst under mild conditions. This method was successfully applied in the process of scale-up synthesis of medicinal candidate product EMB-3.
Introduction
Since Ma et al. reported the first effective ligand amino acid (L1, Fig. 1) for copper catalysis in the synthesis of enantiopure N-aryl-α-amino acids from R-amino acids with aryl halides in 1998,1,2 Brønsted bases,3,4 phenanthroline (L2, Fig. 1),5 1,2-diamino-cyclohexane (L3, Fig. 1)6–8 and several other representative ligands (L4–L7, Fig. 1) have been reported as effective ligands in the CuI-catalyzed aryl amination.9 Based on these novel ligands, many chemoselective methods, such as Csp2- or Csp3-N-arylation,10 Csp2-S-arylation11 and Csp2-O-arylation12 have been studied. However, only a few reported copper catalyst systems could facilitate the coupling between aryl halides and aliphatic alcohols13 because of the weak nucleophilic ability of aliphatic alcohols. For example, researchers, including Buchwald et al., reported a highly efficient phenanthroline ligand (L2, R1, R2, R3, R4 = Me, Fig. 1) in the amination of aryl iodides under mild conditions.14–16Avoiding the use of different complex and expensive ligands, “ligand-free” copper catalyst systems have been reported recently in the O-arylation of aliphatic alcohols (17–59% yields, Scheme 1a).17 However, the reaction substrate was very narrow and high temperatures were required. In addition, the yield of desired ethers was very low and was just determined exclusively using 1H-NMR spectroscopy. Maiti reported an efficient ligand-free Cu-catalyzed O-arylation of aliphatic alcohols 4 and aryl iodide 2 to produce alkyl aryl ether 5 in the presence of 2.3 equivalents of NaOt-Bu (Scheme 1b).18 Although this “ligand-free” methodology was further tested in the one-step synthesis of 2-(2-(4-fluorophenoxy)ethyl)-phenol (CRE 10904: 2-OH, n = 1, R = 4-F, Scheme 1b), it could not be transposed on industrial scale because of its relative low yield of 50%. Very recently, Chae reported a Cu-catalyzed O-arylation of aliphatic alcohols with aryl bromide as substrate and CuCl2 as catalyst (83–99% yields). However, this protocol was effective for the aryl bromide and the required temperature (at 130 °C) was high.19 Therefore, ligand-free Cu-catalyzed O-arylation of aliphatic alcohols remains a challenge.
Our research group has engaged in metal-catalyzed coupling transformation including C–C coupling reactions20 and C–S coupling reactions.21 For the purpose of extending to C–O coupling reactions, the efficiency of ligand-free copper-catalyzed Csp3-O-alkyl chain was investigated. Herein, we disclose a simple and practical ligand-free procedure for the copper-catalyzed arylation of different primary and secondary aliphatic diols (Scheme 1c).
Results and discussion
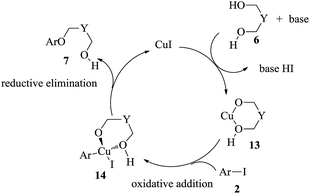
4-Fluoro-iodobenzene 2a and 1, 4-butanediol 6a were selected as model substrates in the experiment under various conditions (Table 1). After 2a was treated with 6a (3.0 equiv.) in the presence of CuI (5 mol%) and NaOt−Bu (3.0 equiv.) in N,N-dimethylformamide at 70 °C for 18 h, product 7a was isolated with a yield of 76% (Table 1, entry 1). Upon using CuBr as the catalyst, product 7a was obtained in lower yield (Table 1, entry 2). As shown in Table 1 (entries 1, 3–5), the amount of CuI had limited influence on the yield. Entries 6–9 in Table 1 show that NaOt-Bu was essential for the coupling reaction because 7a was not obtained with K2CO3, K3PO4, Cs2CO3 or Et3N. Entries 10–14 in Table 1 also show that solvent effects were significant and product 7a could not be produced in THF, DMSO, 1,4-dioxane, MeCN or toluene instead of DMF. When reaction temperature was increased to 80 °C from 70 °C, the yield of 7a was unchanged, but a further increase over 80 °C evidently decreased the yields (Table 1, entries 15–18). When the dosage of diol was increased to 5.0 equiv. or decreased to 1.5 equiv., 7a was isolated in a yield of 78% and 55%, respectively (Table 1, entries 19 and 20). As shown in Table 1, 7a wasn't obtained when CuI wasn't used (entry 22) or when a small amount of water was added (entry 23) or when 1,4-butanediol was replaced by 1-butanol (Table 1, entry 21), suggesting that 1,4-butanediol was the starting material and also the ligand. Therefore, reaction conditions were determined to include CuI (10 mol%) as the catalyst and NaOt-Bu (3.0 equiv.) as the base in DMF at 80 °C with a 2a/6a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 for 18 h (Table 1, entry 15).
3 for 18 h (Table 1, entry 15).
| Entry | Copper | Base | Solvent | Temp. (°C) | Yieldsb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 2a (0.5 mmol), 6a (1.5 mmol, 3.0 equiv.), copper catalyst (0.05–0.2 mmol), base (1.5 mmol, 3.0 equiv.), solvent (2 mL), 18 h.b Isolated yields calculated based on 2a.c 5.0 equiv. 1,4-butanediol was used.d 1.5 equiv. butanediol was used.e 1,4-Butanediol was replaced by 1-butanol.f 4-(4-Iodophenoxy)butan-1-ol 7t instead of 7a was obtained and the structure was confirmed by 19F-NMR, 1H-NMR and 13C-NMR.g 0.5 mL H2O was added to the reaction system. | |||||
| 1 | CuI (5 mol%) | NaOt-Bu | DMF | 70 | 76 |
| 2 | CuBr (5 mol%) | NaOt-Bu | DMF | 70 | 58 |
| 3 | CuI (10 mol%) | NaOt-Bu | DMF | 70 | 77 |
| 4 | CuI (15 mol%) | NaOt-Bu | DMF | 70 | 65 |
| 5 | CuI (20 mol%) | NaOt-Bu | DMF | 70 | 76 |
| 6 | CuI (10 mol%) | K2CO3 | DMF | 70 | 0 |
| 7 | CuI (10 mol%) | K3PO4 | DMF | 70 | 0 |
| 8 | CuI (10 mol%) | Cs2CO3 | DMF | 70 | 0 |
| 9 | CuI (10 mol%) | Et3N | DMF | 70 | 0 |
| 10 | CuI (10 mol%) | NaOt-Bu | THF | 70 | 0 |
| 11 | CuI (10 mol%) | NaOt-Bu | DMSO | 70 | 0f |
| 12 | CuI (10 mol%) | NaOt-Bu | 1,4-dioxane | 70 | 0 |
| 13 | CuI (10 mol%) | NaOt-Bu | MeCN | 70 | 0 |
| 14 | CuI (10 mol%) | NaOt-Bu | Toluene | 70 | 0 |
| 15 | CuI (10 mol%) | NaOt-Bu | DMF | 80 | 78 |
| 16 | CuI (10 mol%) | NaOt-Bu | DMF | 90 | 69 |
| 17 | CuI (10 mol%) | NaOt-Bu | DMF | 100 | 58 |
| 18 | CuI (10 mol%) | NaOt-Bu | DMF | 110 | 67 |
| 19 | CuI (10 mol%) | NaOt-Bu | DMF | 80 | 78c |
| 20 | CuI (10 mol%) | NaOt-Bu | DMF | 80 | 55d |
| 21 | CuI (10 mol%) | NaOt-Bu | DMF | 80 | 0e |
| 22 | CuI (0 mol%) | NaOt-Bu | DMF | 80 | 0f |
| 23 | CuI (10 mol%) | NaOt-Bu | DMF | 80 | 0g |
To further test this reaction, 6a was reacted with various aryl iodides under the optimized reaction conditions. As shown in Table 2, with some electron-withdrawing groups, such as Cl, Br and phenyl, the desired products were obtained in relatively moderate yields (Table 2, entries 4, 5 and 9). However, with other electron-withdrawing groups, such as cyano, trifluoromethyl and benzoyl, the corresponding products were obtained only in very low yield probably due to their strong electron-withdrawing effects (Table 2, entries 6–8). Iodobenzenes bearing one or two electron-donating groups on the phenyl ring, such as 2j, 2k, 2l, 2m, 2n, 2r and 2s reacted with 6a to form the coupled products in low to moderate yields (Table 2, entries 10–14, 18 and 19). In addition to para-substituted iodobenzene 2a and 2l, meta-substituted substrate 2b, ortho-substituted substrate 2c and 2m were also successfully applied to this transformation with relatively low yield (Table 2, entries 2, 3 and 13). Furthermore, iodides with phenyl ring, pyridine ring or thiophene ring gave the desired coupled products (7o: 77%, 7p: 74% and 7q: 53%) without much yield loss (Table 2, entries 15–17). When reaction temperature was decreased to 70 °C from 80 °C, product 7l and 7o were obtained in slightly lower yield (73 and 74% respectively), proving 80 °C was more efficient than 70 °C (Table 2, entries 12 and 15).
| Entry | Ar (Het) | 7 | Yieldsb (%) |
|---|---|---|---|
| a Reaction conditions: 2 (0.5 mmol), 6a (1.5 mmol, 3.0 equiv.), CuI (0.05 mmol, 10 mol%), NaOt-Bu (1.5 mmol, 3.0 equiv.), DMF (2 mL), 80 °C, 18 h.b Isolated yields calculated based on 2.c At 70 °C.d CuI was not added. | |||
| 1 | 2a 4-F-C6H4 | 7a | 78 |
| 2 | 2b 3-F-C6H4 | 7b | 68 |
| 3 | 2c 2-F-C6H4 | 7c | 58 |
| 4 | 2d 4-Br-C6H4 | 7d | 82 |
| 5 | 2e 4-Cl-C6H4 | 7e | 80 |
| 6 | 2f 4-CN-C6H4 | 7f | 30 |
| 7 | 2g 4-CF3-C6H4 | 7g | 44 |
| 8 | 2h 4-Bz-C6H4 | 7h | 38 |
| 9 | 2i 4-Ph-C6H4 | 7i | 64 |
| 10 | 2j 4-NHAc-C6H4 | 7j | 35 |
| 11 | 2k 4-OMe-C6H4 | 7k | 63 |
| 12 | 2l 4-Me-C6H4 | 7l | 78 (73c) |
| 13 | 2m 2-Me-C6H4 | 7m | 58 |
| 14 | 2n 4-OCF3-C6H4 | 7n | 70 |
| 15 | 2o C6H5 | 7o | 77 (74c) |
| 16 | 2p 2-Pyridinyl | 7p | 74 (Traced) |
| 17 | 2q 3-Thiophenyl | 7q | 53 |
| 18 | 2r 3,5-Dimethyl-C6H3 | 7r | 72 |
| 19 | 2s 2,4-Dimethoxyl-C6H3 | 7s | 49 |
When various diols, including aliphatic diols 6b–e and methyl or benzyl substituted diethanol amine 6f–g, were used, the desired products were obtained in 45–86% yields (Table 3, entries 1–9). Compared with 6a, aliphatic diols 6b–e gave the corresponding products 8a–d in lower yields (Table 3, entries 1–4), which indicated that the chain length of aliphatic diols might affect the reaction efficiency. Comparing between N-methyl diethanol amine 6f and N-benzyl diethanol amine 6g, which had comparable reactivities as 6a, 6g exhibited higher reactivity with better yields (Table 3, entries, 5 and 6). Aryl iodides 2a, 2o and 2r reacted with 6f or 6g to afford the desired products in 52–77% yields (Table 3, entries 7–9).
| Entry | 2 | 6 | 8 | Yieldb [%] |
|---|---|---|---|---|
| a Reaction conditions: 2 (0.5 mmol), 6 (1.5 mmol, 3.0 equiv.), CuI (0.05 mmol, 10 mol%), NaOt-Bu (1.5 mmol, 3.0 equiv.), DMF (2 mL), 80 °C, 18 h.b Isolated yields calculated based on 2. | ||||
| 1 | 2l | 6b | 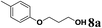 |
59 |
| 2 | 2l | 6c |  |
60 |
| 3 | 2l | 6d | 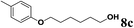 |
61 |
| 4 | 2l | 6e |  |
45 |
| 5 | 2l | 6f |  |
76 |
| 6 | 2l | 6g |  |
86 |
| 7 | 2o | 6f |  |
52 |
| 8 | 2a | 6g | 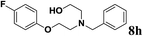 |
77 |
| 9 | 2r | 6g |  |
65 |
To further examine the scope of diols, 2,5-hexanediol 9 was tested under the optimized conditions. As shown in Table 4, iodobenzene derivatives containing electron-donating or electron-withdrawing groups on the aryl moiety reacted with 2,5-hexanediol to produce the corresponding products in 36–78% yields, which indicated that the steric hindrance on diols had limited impact on the reaction.
| Entry | Ar (Het) | 10 | Yieldc [%] |
|---|---|---|---|
| a Reaction conditions: 2 (0.5 mmol), 9 (1.5 mmol, 3.0 equiv.), CuI (0.05 mmol, 10 mol%), NaOt-Bu (1.5 mmol, 3.0 equiv.), DMF (2 mL), 80 °C, 18 h.b Mixture of isomers.c Isolated yields calculated based on 2. | |||
| 1 | 2a 4-F-C6H4 | 10a | 47 |
| 2 | 2k 4-OMe-C6H4 | 10b | 56 |
| 3 | 2l 4-Me-C6H4 | 10c | 64 |
| 4 | 2o C6H5 | 10d | 51 |
| 5 | 2p 2-pyridinyl | 10e | 54 |
| 6 | 2r 3,5-dimethyl-C6H3 | 10f | 78 |
| 7 | 2m 2-Me-C6H4 | 10g | 36 |
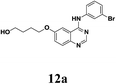
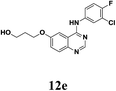
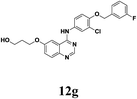
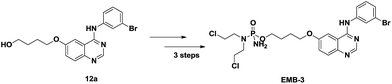
According to Maiti's excellent work, the ligand-free Cu-catalyzed chemoselective mono-arylation of aliphatic alcohols could be applied to modify Ullmann coupling reaction between diols 6 and aryl iodides 11 from commercial available 4-chloro-6-iodo-quinazoline and different anilines, thus to provide [4-phenylamino-6-quinazolinyl]-oxyl-propanol 12, a key intermediate of anticancer drug candidate EMB-3.22,23 Under the optimized conditions, 11a–c reacted with aliphatic diols 6a–c to form the corresponding compounds successfully in 60–82% yields (Table 5, entries 1–7). And this intermediate 12 could shorten the synthesis steps of EMB-3 from 6 to 3. Furthermore, under these optimized reaction conditions, 200 g – scale synthesis (yield: 82%) of 12a, which was a key intermediate of anti-tumor compound EMB-3, was realized.
On the basis of the above results and literature reports,24 we formulated a possible mechanism for the copper-catalyzed tandem cyclization in Scheme 2. In the presence of a base, the chelation of CuI with diols 6 forms a reactive species 13. In this process of forming intermediate 13, diols 6 act as reactant and ligand. The ring strain of intermediate 13 is not supposed to be too strong. Herein, glycol could not react with CuI to form the transition state. Subsequent oxidative addition of intermediate 13 with aryl iodides 2 leads to the intermediate 14. Then CuI is regenerated by a putative reductive elimination, giving the desired products 7 simultaneously.
Conclusions
In summary, we have successfully developed a ligand-free Cu-catalyzed protocol to synthesize alkyl aryl ethers from multi-substituted aryl iodides and aliphatic diols under mild conditions with moderate to good yields. Furthermore, with this method, under the optimized reaction conditions, 200 g – scale synthesis (yield: 82%) of a key intermediate of medicine EMB-3 was realized.Acknowledgements
This research was supported by Ministry of Science and Technology of China (Grant 2012ZX09103101–042).Notes and references
- D. Ma, Y. Zhang, J. Yao, S. Wu and F. Tao, J. Am. Chem. Soc., 1998, 120, 12459–12467 CrossRef CAS.
- D. Ma and Q. Cai, Acc. Chem. Res., 2008, 41, 1450–1460 CrossRef CAS PubMed.
- (a) F. Ullmann and J. Bielecki, Ber. Dtsch. Chem. Ges., 1901, 34, 2174–2185 CrossRef CAS PubMed; (b) F. Ullmann, Ber. Dtsch. Chem. Ges., 1903, 36, 2382–2384 CrossRef PubMed.
- I. Goldberg, Ber. Dtsch. Chem. Ges., 1906, 39, 1691–1692 CrossRef PubMed.
- H. B. Goodbrand and N.-X. Hu, J. Org. Chem., 1999, 64, 670–674 CrossRef CAS.
- A. Klapars, J. C. Antilla, X. Huang and S. L. Buchwald, J. Am. Chem. Soc., 2001, 123, 7727–7729 CrossRef CAS.
- D. S. Surry and S. L. Buchwald, Chem. Sci., 2010, 1, 13–31 RSC.
- F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2008, 47, 3096–3099 CrossRef CAS PubMed.
- K. Okano, H. Tokuyama and T. Fukuyama, Chem. Commun., 2014, 50, 13650–13663 RSC.
- (a) W. Yang, Y. Long, S. Zhang, Y. Zeng and C. Qian, Org. Lett., 2013, 15, 3598–3601 CrossRef CAS PubMed; (b) K. Kunz, U. Scholz and D. Ganzer, Synlett, 2003, 2428–2439 CrossRef CAS; (c) I. P. Beletskaya and A. V. Cheprakov, Coord. Chem. Rev., 2004, 248, 2337–2364 CrossRef CAS PubMed; (d) F. Y. Kwong and S. L. Buchwald, Org. Lett., 2003, 5, 793–796 CrossRef CAS PubMed; (e) D. W. Ma, Q. Cai and H. Zhang, Org. Lett., 2003, 5, 2453–2455 CrossRef CAS PubMed; (f) Z. K. Lu, R. J. Twieg and S. P. D. Huang, Tetrahedron Lett., 2003, 44, 6289–6292 CrossRef CAS.
- (a) C.-F. Lee, Y.-C. Liu and S. S. Badsara, Chem.–Asian J., 2014, 9, 706–722 CrossRef CAS PubMed; (b) F. Y. Kwong and S. L. Buchwald, Org. Lett., 2002, 4, 3517–3520 CrossRef CAS PubMed; (c) M. S. Kabir, M. Lorenz, M. L. van Linn, O. A. Namjoshi, S. Ara and J. M. Cook, J. Org. Chem., 2010, 75, 3626–3643 CrossRef CAS PubMed; (d) Y.-J. Chen and H.-H. Chen, Org. Lett., 2006, 8, 5609–5612 CrossRef CAS PubMed; (e) N. R. Jogdand, B. B. Shingate and M. S. Shingare, Tetrahedron Lett., 2009, 50, 6092–6094 CrossRef CAS PubMed; (f) S. Murru, H. Ghosh, S. K. Sahoo and B. K. Patel, Org. Lett., 2009, 11, 4254–4257 CrossRef CAS PubMed; (g) Y. Feng, X. Zhao, J. Wang, F. Zheng and H. Xu, Chin. J. Chem., 2009, 27, 2423–2425 CrossRef CAS PubMed; (h) R. Xu, J.-P. Wan, H. Mao and Y. Pan, J. Am. Chem. Soc., 2010, 132, 15531–15533 CrossRef CAS PubMed; (i) R.-S. Xu, L. Yue and Y.-J. Pan, Tetrahedron, 2012, 68, 5046–5052 CrossRef CAS PubMed; (j) X. Lv and W. Bao, J. Org. Chem., 2007, 72, 3863–3867 CrossRef CAS PubMed.
- (a) A. Tlili, F. Monnier and M. Taillefer, Chem.–Eur. J., 2010, 16, 12299–12302 CrossRef CAS PubMed; (b) M. Wolter, G. Nordmann, G. E. Job and S. L. Buchwald, Org. Lett., 2002, 4, 973–976 CrossRef CAS PubMed; (c) Also see: G. Nordmann and S. L. Buchwald, J. Am. Chem. Soc., 2003, 125, 4978–4979 CrossRef CAS PubMed.
- (a) H. Zhang, D. Ma and W. Cao, Synlett, 2007, 243–246 CrossRef CAS; (b) R. Hosseinzadeh, M. Tajbakhsh, M. Mohadjerani and M. Alikarami, Synlett, 2005, 1101–1104 CrossRef CAS.
- (a) A. Shafir, P. A. Lichtor and S. L. Buchwald, J. Am. Chem. Soc., 2007, 129, 3490–3491 CrossRef CAS PubMed; (b) R. A. Altman, A. Shafir, A. Choi, P. A. Lichtor and S. L. Buchwald, J. Org. Chem., 2008, 73, 284–286 CrossRef CAS PubMed; (c) G. O. Jones, P. Liu, K. N. Houk and S. L. Buchwald, J. Am. Chem. Soc., 2010, 132, 6205–6213 CrossRef CAS PubMed.
- Y.-J. Jian and Y. Wu, Tetrahedron, 2010, 66, 637–640 CrossRef CAS PubMed.
- F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2009, 48, 6954–6971 CrossRef CAS PubMed.
- (a) I. Gueell and X. Ribas, Eur. J. Org. Chem., 2014, 3188–3195 CrossRef CAS PubMed; (b) J. W. W. Chang, S. Chee, S. Mak, P. Buranaprasertsuk, W. Chavasiri and P. W. H. Chan, Tetrahedron Lett., 2008, 49, 2018–2022 CrossRef CAS PubMed.
- D. Maiti, Chem. Commun., 2011, 47, 8340–8342 RSC.
- Y. Liu, S. K. Park, Y. Xiao and J. Chae, Org. Biomol. Chem., 2014, 12, 4747–4753 CAS.
- (a) Q. Sun, F. Suzenet and G. Guillaumet, Tetrahedron Lett., 2012, 53, 2694–2698 CrossRef CAS PubMed; (b) Q. Sun, F. Suzenet and G. Guillaumet, J. Org. Chem., 2010, 75, 3473–3476 CrossRef CAS PubMed; (c) Y. Wu, Y. Xing, J. Wang, Q. Sun, W. Kong and F. Suzenet, RSC Adv., 2015, 5, 48558–48562 RSC.
- D. Xiao, L. Han, Q. Sun, Q. Chen, N. Gong, Y. Lv, F. Suzenet, G. Guillaumet, T. Cheng and R. Li, RSC Adv., 2012, 2, 5054–5057 RSC.
- R. Li, Z. Ge, S. Lin, R. Li, Q. Sun, X. Wang and T. Cheng, Preparation method and application of antitumor compounds with multiple target points, CN 102850397 A 20130102, Faming Zhuanli Shenqing, 2013.
- Unpublished results.
. - C. Sambiagio, S. P. Marsden, A. J. Blacker and P. C. McGowan, Chem. Soc. Rev., 2014, 43, 3525–3550 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra12529d |
| This journal is © The Royal Society of Chemistry 2015 |