Magnetic chemiluminescent immunoassay for human C-reactive protein on the centrifugal microfluidics platform†
Abstract
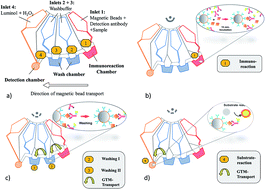
Human C-reactive protein (CRP) has been reported as an inflammatory biomarker with the highest reference for use in clinical practice. However, the existing analytical techniques are lacking automation and simplicity, as desired for a prospective immunoassay format for point-of-care (PoC) analysis. We have developed an automated magnetic chemiluminescent immunoassay (MCIA) on a mobile analyser for rapid PoC determination of CRP. The MCIA is fully automated after the initial loading of sample and immunoreagents at the inlet ports. The automated protocol involves the transportation of magnetic capture microparticles between adjacent reaction compartments using a set of stationary magnets, a microfluidic polymer disposable and a specific centrifugal protocol. The developed MCIA has a sample-to-answer time of 25 min and hands-on time of approximately 5 min. It detects the entire pathophysiological range of CRP, as desired for clinically-relevant high sensitivity CRP immunoassay format, i.e. 3–81 ng mL−1 in diluted human serum with a limit of detection (LOD) and limit of quantification (LOQ) of 1.5 ng mL−1 and 1.8 ng mL−1, respectively.

 Please wait while we load your content...
Please wait while we load your content...