Fabrication of nanocomposites by covalent bonding between noble metal nanoparticles and polymer matrix†
M. Iwan,
T. Andryszewski,
M. Wydryszek and
M. Fialkowski*
Institute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland. E-mail: mfialkowski@ichf.edu.pl
First published on 10th August 2015
Abstract
We present a novel approach to the synthesis of polymeric nanocomposites in which noble metal nanoparticles (NP) are covalently bonded to a polymer matrix. In this approach, the NPs are functionalized with novel aminothioalkil ligands that are capable to bind chemically to a wide range of polymers possessing carbonyl groups or hydroxyl groups that can be oxidized into carbonyl groups. This method is illustrated by the ex situ incorporation of gold, platinum, and silver NPs decorated with the aminothioalkil ligands into matrices of natural polymers such as potato starch, cellulose, as well as synthetic polymers – poly(vinyl)alcohol, carboxymethylcellulose, and copolymer of maleic anhydride and styrene. It is demonstrated that in the obtained nanocomposites the functionalized NPs are covalently linked to the polymer matrix via amide, imide, imine, or aminal bonds. We show that the binding reaction of the aminothioalkil ligands with the polymer chains occurs spontaneously at room temperature only when the aminothioalkil ligands are attached to the surface of the NPs.
1. Introduction
Combining nanoparticles (NP) with natural or synthetic polymer matrix opens a path to obtaining novel nanocomposite materials that possess advantageous qualities. Properties of such materials – obtained either via incorporation of the NPs into the matrix or in situ synthesis of the NPs within the matrix – are determined by a combination of many factors, such as the chemical composition, density, shape, arrangement of the inclusions, as well as the constitutive properties of the host material.1 Incorporation of NPs into polymeric matrices often gives rise to substantial improvement in the mechanical,2 thermal,3 and electrical4 properties of the host material. Strategies employed currently for the incorporation of the NPs into polymer matrix involve either in situ or ex situ synthesis of the NPs.5,6 The NPs can be embedded in the bulk of the polymer matrix, which is a typical situation when the NPs are formed in situ simultaneously with the matrix, or bound on or near the surface, which occurs when the NPs are synthesized ex situ or in situ after the formation of the matrix. The main drawback of the in situ method is the possible influence of byproducts or unreacted substrates, which are not removed after the synthesis, on the nanocomposite properties.7 Moreover, the unique properties of the NPs strongly depend on their size, shape, and surface chemistry.8–10 Thus, preparation of monodispersed NPs of well-defined properties is crucial for the fabrication of nanocomposites with tailored characteristics. This issue is of extreme importance in applications such as catalysis, where the shape and size of the NPs determine their catalytic activity.11,12 In this respect, the ex situ approach provides a better control over the synthesis of the NPs and allows for the transfer of the size dependent features of the NPs into the nanocomposite property profile.The binding manner of the NPs to the matrix has also a significant impact on the properties of the composite material. Strong chemical bonding of the NPs is highly advantageous over weak linkage, since it provides means for permanent attachment of the NPs to the polymer, which prevents their leaching from the composite. The immobilization of the NPs within the host is important not only because it makes the material far more resistant and durable, but also due to the raising concerns about the influence of the NPs on the environment, and the health risks associated with their release.13 Better fixation of the NPs within nanocomposites is one of the approaches employed to the reduction of hazards associated with the release of NPs.14 Robust bonding, that prevents the NP leaching, is of high importance in some of the new applications of the nanocomposites in medicine, like, for example, the fabrication of bactericidal materials.15,16 Another significant advantage of the chemically bound NPs is their lack of tendency towards aggregation in the immobilized state, which may occur due to low compatibility between the NPs and the polymer matrix.17 The aggregation of the NPs is undesired because it can impair functionality of the composite.15,17,18 The high surface area of nanoobjects makes them prone to aggregation at high concentrations,19 which may influence significantly the material properties.20 Functionalization of NP surface with ligands that enhance the compatibility between the NPs and matrix is a recently reported strategy that inhibits agglomeration of NPs.21 However, the weak character of interaction between the NPs and polymeric matrix does not guarantee durability of the obtained nanocomposite. Thus, strong enough attractive interaction between the NPs and polymer must be provided to prevent the aggregation. For this reason, covalent bond formation between the NPs and the polymer matrix are desirable22 for fabrication of highly-loaded nanocomposites.
In typical fabrication strategies, the NPs are weakly bound to the matrix via hydrogen23 or coordination bonds,24 electronic,25 or electrostatic attraction between the polymer and the functionalized NPs.26 So far, there are very few reports on methods allowing chemical (covalent) bonding of the NPs to the host.27,28 These methods utilize chemically modified matrices, where the surface of the matrix contains functional groups of high affinity that act as anchors for the in situ synthesized NPs.27 However, the above approach is not robust because it relies on specific chemical reaction and requires selection of the reaction conditions for each type of the polymer host employed. Recently, a novel approach to the fabrication of nanocomposite containing covalently bonded ex situ synthesized NPs has been reported.28 In the work cited, the reaction of formation of the covalent bonds proceeds through radical generation, and is initiated by the UV irradiation. Radical reactions are, however, known to proceed rapidly, in a way that is difficult to control. Grafting polymers from the surface of NPs is also an interesting approach, in which surface-modification of NPs with groups capable of initiating polymerization is employed.29 Nonetheless, this process may be difficult to control if two reactive radicals are formed from one initiating molecule. The polymerization may then proceed both on the surface of the NPs and in the bulk. Controlled radical polymerization is a solution, but high concentrations of transition-metal catalysts are required in this case. These metals contaminate the final product and their recovery is difficult. Other examples of covalent attachment include grafting the surface of nanofillers with polymerizable groups, which may be used to obtain both linear and cross-linked composites.29 However, due to the interaction of the polymerizing agents with other molecules that may be present in the system control over the final product is difficult. Having this in mind, it is clear that developing a more facile and versatile method of the ex situ synthesis of covalently bonded polymer-NPs nanocomposites would be beneficial.
Here, we present a novel approach to the chemical linking of ex situ prepared noble metal NPs to various polymer matrices (Fig. 1). Our method utilizes a binding reaction between the NPs functionalized with aminothioalkil ligands and polymers containing any of the following groups in their structure: the aldehyde, carboxyl acid, or anhydride acid group. The binding can be also carried out when the polymer matrix possess hydroxyl groups that can be oxidized to carbonyl groups in the reaction conditions. The capability of linking the NPs through different functional polymer groups makes this method potentially versatile with respect to the type of the host matrix. Additionally, the presence of the thiol group in the terminal position of the ligand structure renders these molecules potential capping ligands for a variety of metallic and semiconducting particles.
 | ||
| Fig. 1 Schematic illustration of the nanocomposite fabrication via spontaneous covalent bond formation between NPs' capping ligands and polymer functional groups. | ||
2. Experimental
2.1 Materials
Acetonitrile, methanol, n-hexane, hydrazine solution in water, chloroform and acetone were purchased from POCH. Potassium phthalimide, 1,4-dibromobutane, 1,8-dibromooctane, 1,12-dibromododecan, potato-starch and hydrochloric acid (38%), tri-sodium citrate, HAuCl4 ⋅ 3H2O, K2PtCl4, AgNO3, sodium borohydride were purchased from Aldrich. Potassium thioacetate was purchased from Alfa Aesar. Poly(vinyl alcohol) (95% hydrolyzed, average MW 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was purchased from Acros Organics.
000) was purchased from Acros Organics.
2.2 Synthesis of the aminothiolalkil ligands
Scheme 1 shows synthetic routes and structures of the novel aminothioalkil ligands designed for the functionalization of the NPs. The synthesis of these ligand was simple and consisted of only three steps. In the first step, one of the bromine atoms of dibromoalkane (e.g. 1,8-dibromooctane) was substituted by the phthalimide group. Then, the second bromine atom was replaced by the thioacetate group. Finally, the terminal protective phthalimide and acetate groups were removed in a deprotection reaction (hydrazinolysis and hydrolysis with hydrazine and hydrochloric acid, respectively).2.3 Synthesis of the P[MA-co-S] copolymer
During the synthesis of the P[MA-co-S] copolymer 2.943 g (30.0 mmol) of maleic anhydride and 3.100 g (29.77 mmol) of styrene were added into 30 mL of chloroform in a round-bottom flask. The obtained mixture was then stirred at room temperature until complete dissolution of maleic anhydride. The mixture was then heated to boiling temperature and subsequently 0.600 g (3.654 mmol) of the radical reaction initiator – azobisisobutyronitrile (AIBN) was added. The mixture was stirred at boiling temperature for 4 h. During this time the solution remained clear and colourless, but a pronounced increase of the viscosity of the solution was observed. The reaction mixture was then cooled to room temperature and transferred into a plastic syringe. Finally, the solution was added drop-wise into 200 mL of methanol upon intensive stirring. A white precipitate of the final product was formed. The obtained mixture was then filtered and the precipitate was washed with 200 mL of methanol. As a result, 5.156 g (25.53 mmol) of the copolymer was obtained (reaction yield: 85.75%).2.4 Synthesis of gold, silver, and platinum NPs
In our experiments, we used gold and silver NPs that were synthesized according to the literature procedures.30,31 In brief, to obtain AuNPs, a basic solution of sodium borohydride (3 mL; 50 mM) was added to an acidic aqueous solution of chloroauric acid (1 mL; 50 mM) in water (100 mL), upon which a change of colour from yellow to dark red was observed, indicating the formation of AuNPs. To obtain AgNPs, tri-sodium citrate solution (1.6 mL; 38.3 mM) was mixed with fresh aqueous sodium borohydride solution (0.4 mL; 112 mM). The obtained solution was cooled to 10 °C and aqueous solution of AgNO3 (20 mL; 1 mM) was added dropwise under continuous stirring, upon which a yellow colour appeared. In the case of PtNPs aqueous solution of K2PtCl4 (7.8 mL; 10 mM) and aqueous solution of tri-sodium citrate (3.64 mL; 34 mM) were added to distilled water (65 mL). Next fresh aqueous sodium borohydride solution was prepared by dissolving sodium borohydride (4.17 mg) in tri-sodium citrate solution (5.55 mL; 34 mM) and diluting the obtained mixture with distilled water (25 mL). Finally, the obtained NaBH4 solution was added dropwise under continuous stirring to the solution of K2PtCl4 at room temperature. The obtained mixture was kept at room temperature for 5 h and then was centrifuged at 9000 rpm during 10 min. The obtained supernatant was separated giving a clear, greenish solution of PtNPs.To functionalize AuNPs, 2 mL of distilled water, aqueous solution of 8-mercaptooctane-1-aminium chloride ligand (171 μL; 10 mM) and hydrochloric acid (96 μL; 0.8 M) were added into a glass vial. The solution was then heated to 70 °C, and a solution of AuNPs (20 mL) was added drop-wise into the flask at the rate of 1 drop per second. The mixture was kept at 70 °C for one hour. The obtained NPs were stable for more than 3 months. The NPs were centrifuged at 9000 rpm for 10 min to remove all objects larger than 30 nm.
To functionalize the AgNPs, an acidic solution of the ligand was prepared by mixing glacial acetic acid (50 μL), aqueous solution of the ligand (50 μL; 0.001 M) and of distilled water (1 mL). This solution was next cooled to 3–5 °C, and AgNPs solution (5 mL) was added. The colour of the solution should not change upon the addition of the AgNPs. The obtained AgNPs were stable for several hours in the solution.
To functionalize PtNPs, distilled water (2.2 mL), aqueous solution of 8-mercaptooctane-1-ammonium chloride ligand (190 μL; 10 mM) and hydrochloric acid (0.6 μL; 0.8 M) were added into a glass vial. The mixture was then heated to 70 °C in a water bath, and 2 mL of the aqueous solution of PtNPs was added drop-wise at 0.5 mL min−1 rate. The mixture was kept at the elevated temperature for another 10 min.
2.5 Preparation of the starch-based composites
To obtain the powder starch–AuNPs nanocomposite, to 0.5 g of potato starch 5 mL of distilled water was added at room temperature. Then 10 mL of aqueous solution of AuNPs (10−8 mol L−1) functionalized with aminothioalkil ligands was injected under continuous stirring. Once the sediments settled on the bottom of the vial, the red colour of the solution vanished and the solution became colourless. At the same time, the white precipitate of starch turned pink. The clear solvent was then decanted and the remaining residue, washed three times with distilled water and then left to dry at 55 °C for 12 h and then 85 °C for another 3 h.To obtain the free-floating starch–AuNPs nanocomposite film, to 20 mL of distilled water heated up to 85 °C 1 mL of starch suspension (0.1 g mL−1) was added under intensive stirring. The obtained solution was stirred for 1 h at elevated temperature. During this time the solution gradually become uniform and lucid. Into another vail 5 mL of AuNPs functionalized with aminothioalkil ligand (AuNP@C4, AuNP@C8) was added, and the solution was heated to 85 °C. Then, 0.5–1 mL of the clear starch solution prepared in the previous step was added and the obtained mixture was stirred at 85 °C for another 10 min. Next, the sample was cooled to room temperature and 3 mL of methanol were added dropwise, upon which clouding of the solution was observed. The mixture was then centrifuged at 9000 rpm for 15 min. Next, the supernatant was removed, and to the remaining residue (the starch-NPs film) methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixtures of the ratios (1
water mixtures of the ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), (3
1), (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and (1
1), and (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) (v/v) were subsequently added. The mixtures of increasing methanol content were employed to change gradually the polarity of the solvent and facilitate peeling off the film from the vial walls. After each addition of the methanol
0) (v/v) were subsequently added. The mixtures of increasing methanol content were employed to change gradually the polarity of the solvent and facilitate peeling off the film from the vial walls. After each addition of the methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixture the obtained solution was left for 0.5 h at room temperature. Finally, the starch-NPs film formed during the centrifugation was detached from the polymer vial walls.
water mixture the obtained solution was left for 0.5 h at room temperature. Finally, the starch-NPs film formed during the centrifugation was detached from the polymer vial walls.
2.6 Preparation of the PVA-based composites
To obtain the PVA-based nanocomposites, an aqueous solution of PVA was prepared (50 mg mL−1) by dissolving 250 mg of the polymer in 5 mL of water heated up to 70 °C. 3 mL of AuNP@C8 aqueous solution (10−8 mol L−1) was then added to the PVA solution and the mixture was stirred for 10 min at 70 °C. Finally, the obtained solution was poured into 50 mL of methanol upon which a precipitate of the composite was obtained.The PVA–AuNPs free-standing film was prepared by dissolving 2.5 g of PVA in 20 mL of aqueous solution of AuNPs functionalized with aminothioalkil ligand (AuNP@C8, AuNP@C12) acidified with 0.4 mL 0.8 M hydrochloric acid. The obtained solution was then heated to 75 °C and kept at this temperature for 20 min under continuous stirring. After this time the solution was centrifuged at 5000 rpm for 15 min. To 3 mL of the obtained supernatant 0.5 mL of 86% glycerin was added. Next, the solution was poured onto a Petri dish and dried at 60 °C during 24 h. The obtained film could be easily peeled off the Petri dish with tweezers.
2.7 Preparation of the P[MA-co-S]-based composites
The P[MA-co-S]-based composite was obtained by adding 5 mL of AuNP@C8 aqueous solution (10−8 M) to 10.5 mg of the polymer. The polymer did not dissolve in the aqueous solution of the AuNPs, but gradual lightening of the initial red colour of the solution was observed, indicating the attachment of the AuNPs to the surface of the solid polymer. After 12 h the reddish precipitate was decanted and washed thrice with distilled water to remove any unbound NPs.2.8 Preparation of the cellulose-based composites
During the preparation of the cellulose-based composite a piece of cellulose paper was cut (165 mg) and placed in a glass vail. 5 mL of AuNP@C8 aqueous solution (10−8 M) was added into the vial and the obtained mixture was left for 12 h. During this time gradual lightening of the red colour of the solution was observed due to the attachment of the NPs to the surface of the paper. At the same time, the colour of the paper strip turned pink. After 12 h the paper was removed from the solution. After immersing this paper in distilled water the solution remained clear and colourless which confirmed that the detachment of the NPs did not occur.2.9 Preparation of the carboxylmethylcelullose-based composites
To conduct the binding of the metallic NPs (Au@C8) to the carboxylmethylcelullose (CMC), 156.7 mg of CMC was added to 10 mL of an aqueous solution of Au@C8 (10−8 M) under continuous stirring. After 30 min the mixture was poured onto a hydrophobic Petri dish and left until complete evaporation of the solvent at 70 °C after the water evaporation the obtained film was peeled off.2.10 Sample preparation for IR measurements
Because the PVA polymer has a neutral charge and some of its functional groups are in the form of acetyl esters after its synthesis, it was necessary to hydrolyze the ester bonds before the functionalization process of the polymer matrix with the NPs. To hydrolyze these bonds the polymer was subjected to an aqueous base solution, which allowed for the removal of the acetyl groups and change of the surface charge of the polymer, from neutral to negative. This facilitated the chemical bonding of the NPs, since they could migrate to the vicinity of the PVA surface due to attractive electrostatic interactions.2.11 PVA functionalized with metallic NPs coated with C8 ligand (Ag@C8, Au@C8, Pt@C8)
To functionalize PVA with metallic NPs, 10 mL of methanol and 1 mL of aqueous NaOH (0.4 M) were added to 100 mg of PVA polymer. The obtained suspension was kept at room temperature for 15 min, then the supernatant was removed, and to the remaining precipitate again 10 mL of methanol and 1 mL of NaOH (0.4 M) were added. The suspension was heated in a water bath at 50 °C during 3 min and then kept at room temperature for another 10 min.After this time the supernatant was removed. In the next steps, appropriate solution of NPs functionalized with C8 ligand were added to the basified PVA polymer. For PtNPs, the solution of Pt@C8 was added gradually by adding in subsequent steps (a) 10 mL methanol, 1 mL of Pt@C8 and 0.6 mL NaOH (aq.) (0.4 M) (b) 10 mL methanol, 1 mL Pt@C8, 0.6 mL of NaOH (aq.) (c) 10 mL methanol, 1 mL Pt@C8, 0.8 mL NaOH (aq.) (0.4 M) (d) 10 mL methanol, 1 mL Pt@C8, 0.6 mL NaOH (aq.) (0.4 M). Each time the resulting solutions were slightly basic. For AgNPs and AuNPs, the NPs were gradually added by repeated addition of 10 mL of methanol, 2 mL of aqueous solution of metallic NPs (Ag@C8 or Au@C8) and 0.2 mL of aqueous NaOH solution (0.4 M) (in case of first two repetitions), letting the solution set for 10 min, before centrifugation at 9000 rpm during 5 min and subsequent removing of the supernatant. This procedure was repeated 17 times for Au@C8, and 20 times for Ag@C8. After the addition of Au@C8, Ag@C8, or Pt@C8 the obtained solution was kept for 10 min at room temperatures to allow the NPs to bind with the matrix. Gradual vanishing of the red (Au@C8), orange (Ag@C8) or greenish (Pt@C8) color of the solution and darkening of the precipitate accompanied this process. Finally, the obtained dark red PVA–Au@C8, dark grey PVA–Ag@C8 or greenish PVA–Pt@C8 precipitate was washed three times with 10 mL of methanol. The precipitate was dried at 3 h under vacuum at room temperature. Half of the obtained PVA–Au@C8 was then sintered at 80 °C during 12 h.
The PtNPs solution required concentration before the functionalization. To carry out the functionalization, 1 mL of aqueous solution of C8 ligand (10 mM) was acidified with hydrochloric acid (0.8 M). The obtained solution was heated to 80 °C in a water bath and 3 mL of the concentrated solution of PtNPs were gradually added, in 100 μL portions.
2.12 PVA functionalized with unbound aminothiolalkil ligand C8
An aqueous solution of aminothioalkil C8 (10 mM) was prepared in the first step by mixing together 3.9 mg of 8-aminooctan-1-thiol in the form of hydrochloride salt, 2 mL of distilled water, and 25 μL of aqueous HCl solution (0.8 M). Then, from the obtained solutions, mixtures for the functionalization of PVA were prepared by mixing 22 mL of distilled water, 171 μL the C8 ligand solution (10 mM) prepared in the previous step, and 96 μL of aqueous solution of HCl (0.8 M). In the next step, PVA was basified by adding 10 mL of methanol and 1 mL of aqueous NaOH (0.4 M) to 100 mg of PVA polymer. The obtained suspension was kept at room temperature for 15 min, then the supernatant was removed and to the remaining precipitate again 10 mL of methanol and 1 mL of NaOH (0.4 M) were added. The suspension was heated in a water bath at 50 °C during 3 min and then kept at room temperature for another 10 min. After this time the supernatant was removed. In the following steps the solution of the C8 ligand was added gradually by adding each time 10 mL of methanol, 2 mL of aqueous solution of amine derivative (C8) and 0.2 mL of aqueous NaOH solution (0.4 M) (in case of first two repetitions), letting the solution set for 10 min, before centrifugation at 9000 rpm for 5 min, followed by removal of the supernatant. This procedure was repeated 17 times. Finally, the obtained white precipitate was washed three times with 10 mL of methanol. The precipitate was dried for 3 h under vacuum at room temperature.2.13 Instrumentation
1H and 13C NMR spectra were recorded on a Varian Gemini spectrometer (200 MHz) and Varian Mercury 400 MHz. UV-vis absorption spectra were recorded on an Evolution 220 by Thermo Scientific company with quartz disposable cuvettes with 1 cm optical path. FTIR spectra were recorded with a FTIR Jasco 6200 spectrometer.3. Results and discussion
3.1 Potato starch
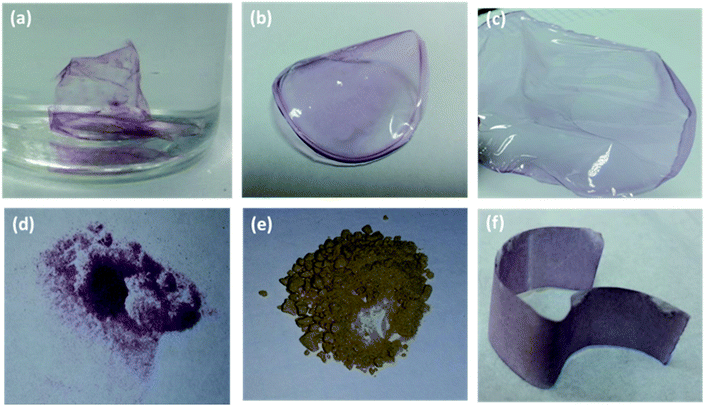
We employed potato starch to synthesize two forms of the nanocomposite material: (i) thin film, and (ii) powder nanocomposite. To obtain the film, we mixed aqueous solution of starch with solution of the AuNPs functionalized with aminothioalkil ligands (AuNP@C4, AuNP@C8) at elevated temperature. Once the bonds between the capping ligands and the functional groups of starch were created, the obtained mixture was centrifuged at high speed. The centrifugation resulted in formation of a robust nanocomposite film on the walls of the vial. The obtained film was then peeled off from the walls and transferred into a vail filled with methanol to demonstrate the strength of bonding between the NPs and the polymer matrix. The resulting starch–AuNPs nanocomposite film floating freely in water is shown in Fig. 2a. Importantly, the Au@C4 were not released from the film into water. Also, as we checked, the synthesized material was durable enough to be taken out (with tweezers) from water and placed on a solid substrate without being torn.To obtain the powder nanocomposite, we applied a different synthetic procedure: solid starch powder was added into a solution of the NPs functionalized with the aminothioalkil ligands. The obtained mixture was then stirred at room temperature until the supernatant became colorless, signaling that all the NPs were consumed. The supernatant was then removed and the remaining precipitate was washed with distilled water. Following this procedure, we successfully incorporated three types of the NPs into the polymer matrix – the AuNPs, PtNPs, and AgNPs, all coated with the same C8 ligand. The materials obtained with the use of AuNPs and AgNPs are shown in Fig. 2d and e, respectively. Importantly, the NPs can be embedded either on the surface or within the bulk of the matrix, depending on the employed protocol of the composite preparation. When the reaction of the bond formation between the capping ligands and the surface groups of starch is carried out at room temperature, the NPs are embedded only on the outer portions of the polymer matrix. This is caused by the low solubility of starch in aqueous environments at temperatures lower than 70 °C.32 Preheating the starch solution to 90 °C prior to the addition of the NPs results in a homogenous embedding of the NPs throughout the bulk of the polymer matrix. SEM images of the starch–AuNP@C8 grain obtained at room temperature shown in Fig. 3 reveal uniform distribution of the NPs on the surface of the nanocomposite (see also Fig. S1†).
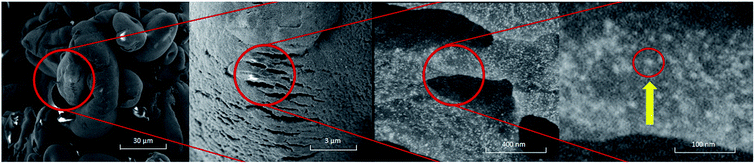 | ||
| Fig. 3 SEM images of the starch–AuNP@C8 powder nanocomposite. The yellow arrow indicates single AuNP bound to the matrix. | ||
The starch modification process is facilitated by electrostatic attraction between the positively charged NPs and the negatively charged starch polymer chains. The positive charge is provided by the capping ligands possessing primary amine groups in the terminal position which are easily protonated. The electrostatic interaction allows the NPs to locate themselves on the polymer chain. The NPs are linked through imine bonds that are formed between the terminal aldehyde groups of the polymer and the amine groups of the capping ligand. Since the aldehyde moieties are present only in the terminal positions of the polymer backbone, the number of the imine bonds formed is expected to be miniscule compared to the total number of the monosaccharide units. The aldehyde group is liberated in the tautomeric transformation between the cyclic and linear form of the terminal glucose unit (Scheme 2b). However, the total number of the aldehyde groups can be substantially increased during the modification process either by acidification or oxidation (Scheme 2a).
In acidic conditions hydrolysis of the 1,4- or 1,6-glycoside bonds occurs, releasing one aldehyde group per each hydrolyzed bond. In the case of oxidation, cleavage of the C–C bonds between the second and third carbon atoms of the glucose ring takes place. Both the hydroxyl groups created are then oxidized yielding two aldehyde groups per each C–C broken bond.33
In the case discussed, acidic hydrolysis is more likely to occur, since the solution of aminothioalkil-functionalized AuNPs is highly acidic. However, the oxidation process can also take place due to oxygen present in the solution. The increase of the number of the aldehyde groups allows incorporation of a greater amount of the NPs, which is beneficial for the nanocomposite preparation process. Importantly, the NPs create interconnections between the polymer chains that can compensate for the linkages lost due to the hydrolysis process. Thanks to these connections the composite material is gaining the mechanical durability. The formation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N imine bonds was confirmed by FTIR spectroscopy performed for the starch–AuNP@C8 nanocomposites. The evidence of the creation of the covalent bonds follows from the comparison of the spectra of the unmodified potato starch and the same polymer modified by the incorporation of AuNP@C8. As can be seen in Fig. S2,† a new peak located at around 1680 cm−1 emerged in the spectrum of the AuNP-modified starch, visible as a widening of the neighboring band. Also, substantial increase of the intensity of the peak located near 1665 cm−1 was observed. This provides a clear evidence of the formation of the imine bonds.34 To demonstrate the durability of the chemical bonds formed between the capping ligands and the polymer matrix in the presence of the NPs, we performed experiments showing that leaching of the NPs form the composite into an aqueous environment does not occur during soaking of the nanocomposite. The NPs were not detached from the surface of the polymer even after ultrasonic exposure of the nanocomposite in aqueous solutions. The lack of leaching was confirmed by UV-vis spectroscopy. To perform the measurements, 45 mg of the starch–AuNP@C8 powder nanocomposite was added into 5 mL of distilled water. After 1 h UV-vis spectrum of the supernatant was recorded. The nanocomposite was then subjected to 1 h of ultrasonic exposure and 24 h of subsequent soaking, and the UV-vis spectrum was taken. Both the obtained spectra were identical and displayed no peaks characteristic for the NPs. This proved that release of the NPs did not take place.
N imine bonds was confirmed by FTIR spectroscopy performed for the starch–AuNP@C8 nanocomposites. The evidence of the creation of the covalent bonds follows from the comparison of the spectra of the unmodified potato starch and the same polymer modified by the incorporation of AuNP@C8. As can be seen in Fig. S2,† a new peak located at around 1680 cm−1 emerged in the spectrum of the AuNP-modified starch, visible as a widening of the neighboring band. Also, substantial increase of the intensity of the peak located near 1665 cm−1 was observed. This provides a clear evidence of the formation of the imine bonds.34 To demonstrate the durability of the chemical bonds formed between the capping ligands and the polymer matrix in the presence of the NPs, we performed experiments showing that leaching of the NPs form the composite into an aqueous environment does not occur during soaking of the nanocomposite. The NPs were not detached from the surface of the polymer even after ultrasonic exposure of the nanocomposite in aqueous solutions. The lack of leaching was confirmed by UV-vis spectroscopy. To perform the measurements, 45 mg of the starch–AuNP@C8 powder nanocomposite was added into 5 mL of distilled water. After 1 h UV-vis spectrum of the supernatant was recorded. The nanocomposite was then subjected to 1 h of ultrasonic exposure and 24 h of subsequent soaking, and the UV-vis spectrum was taken. Both the obtained spectra were identical and displayed no peaks characteristic for the NPs. This proved that release of the NPs did not take place.
Unfunctionalized NPs or NPs functionalized with nonreactive organic ligands, such as carbohydrate chains, do not bind to solid matrices at all or form weak bonds. Binding through the non-chemical bonds (hydrogen bonding, electrostatic interactions, or dispersive forces) results in a nanocomposites from which the NPs quite easily leach into the environment. In our experiments, we observed that the Martin's AuNPs in neutral pH (in basic and acidic conditions they become unstable and aggregate rapidly) hardly adsorbed on potato starch, and the obtained powder nanocomposite had light red color. Moreover, this nanocomposite slightly stained water a reddish color each time it was immersed in it.
3.2 Poly(vinyl)alcohol (PVA)
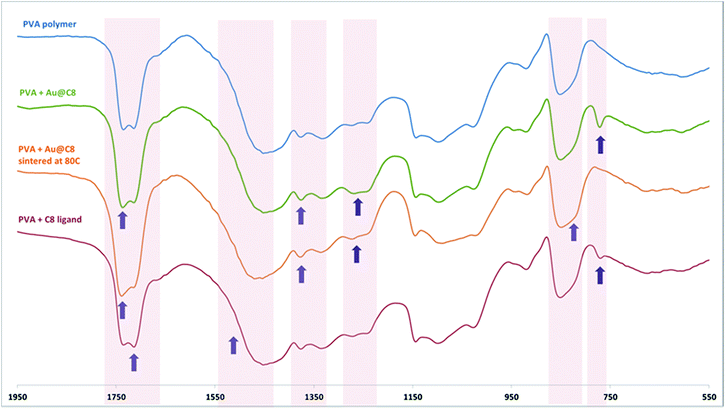
PVA is a widely used synthetic polymer that does not contain carbonyl groups. We employed it successfully to obtain a free standing nanocomposite film. The modification method comprised mixing of aqueous solution of the polymer with a solution of the AuNP@C8 at elevated temperature followed by evaporation of water. The resulting free-standing nanocomposite film is shown in Fig. 2b. The modification of PVA is feasible because, as it has been reported,35 in oxidizing conditions this polymer undergoes degradation resulting in a scission of the main chain. This decomposition process involves formation of polyketones that can be further converted into aldehydes and carboxylic acid terminated polymers (Scheme 2c). This process is accelerated significantly at high temperatures, which is the reason why the functionalization should be carried out at elevated temperatures (70 °C). A peak at around 1760 cm−1 in the FTIR spectrum corresponding to the aldehyde group was observed in the PVA polymer before any processing (see ESI†), indicating that the oxidation process occurs in the solid state at air conditions. The formation of the aldehyde and carboxyl acidic groups allows for the linking of the polymer with the aminothioalkil ligands through amide bonds. The presence of these bonds in the PVA–AuNP@C8 nanocomposite was evidenced by 13C NMR and FTIR spectroscopy.The FTIR spectra of unmodified PVA and PVA–Au@C8 that are shown in Fig. 4 exhibit substantial differences. The comparison of these two FTIR spectra provides a clear evidence for the formation of peptide and imide bonds between PVA functional groups and the ligand amine groups. A considerable intensification of the signals at 1735 and 1240 cm−1 is noticeable. These signals correspond to the imide or secondary amide groups in the solid state.34 The strong band at 1735 cm−1 is a sum of two overlaying bands: a band of the aliphatic secondary amides, which is usually visible as a strong band at 1650–1630 cm−1, and an imide band adsorbing at higher frequencies, up to 1740 cm−1.34 The signal at 1240 cm−1 is characteristic for imides in the solid phase and is due to the C–N stretching of the imide group. An important change in the spectrum after the functionalization is also discernible as a widening of the broad signal at around 1435 cm−1. It is caused by the overlaying of this signal with the characteristic secondary amide band occurring in the band 1565–1475 cm−1.36 Also, widening of the signal at 848 cm−1 is associated with the appearance of a secondary amide band related with the N–H wagging vibration.35 Note that the other characteristic amide bands that are located at around 3400 cm−1 are not visible due to the presence of the wide peak associated with hydroxyl groups in the PVA structure.
The presence of amide bonds was further confirmed by NMR, as a signal at 174 ppm was visible on the spectra (see Fig. S5†). Note that the FTIR spectra of PVA–AgNP@C8 and PVA–PtNP@C8 composites (shown in Fig. S4†) were quite similar to that of the PVA–AuNP@C8, displaying signals of amide and imide bonds.
The FTIR spectrum of the PVA–Au@C8 nanocomposite after sintering at 80 °C was also recorded. As can be seen in Fig. 4, after the exposure to the elevated temperature, the signal at 765 cm−1 that is due to the –NH3+ rocking of amine salts disappears. This signal originates from a fraction of the amine groups that form carboxylate aminium salts with acidic groups. Other characteristic amine salt signals (in the bands 3550–3355, 3350–3150, and 1560–1625 cm−1) are covered by polymer and water bands.
The salt is formed as an intermediate product of the amide formation (see Fig. 6). It follows that some of the amine groups are initially trapped in the form of carboxylate aminium salts, though they may be transformed into amides after thermal energy is delivered to the system. That is, complete transformation of amines into amide groups is possible in the presence of the NPs, but requires heat treatment.
3.3 Carboxymethylcellulose (CMC)
CMC is an anionic water-soluble polymer derived from cellulose that is commonly used in the industry. We applied CMC for the preparation of a free-standing nanocomposite film. Using a method similar to that applied to obtain the PVA-based nanocomposite film, we incorporated the AuNP@C8 into the bulk of the polymer matrix. The synthesis was easy to perform because the capping ligands readily bound to the carboxylic groups of the CMC chain forming imine bonds. The resulting nanocomposite material had a form of a robust semi-transparent free-standing film (∼0.5 mm thick), which is presented in Fig. 2c. The thickness of the film can be easily controlled by the amount of CMC applied onto the Petri dish.3.4 Poly[maleic anhydride-co-styrene] (P[Ma-co-S])
Experiments were also performed using a synthetic copolymer of maleic anhydride and styrene, P[MA-co-S], as the matrix. The modification process was simple, and was done by immersing granules of this polymer in aqueous solution of AuNP@C8. This procedure is illustrated in Fig. 5. The aminothiol molecules readily bound to the polymer due to the presence of acidic anhydride functional groups in the chain (see Scheme 2d). The binding occurs through the formation of aminal and hemiaminal bonds, and was confirmed by 13C NMR studies.It was found that in the NMR spectrum new signals emerged after the modification of the polymer matrix (see Fig. S6†). The new peaks appeared at 81 ppm and in the range from 95 to 104 ppm. The signal located at 81 ppm is characteristic for the aminal bonds, whereas the signals in the higher region are due to the formation of the hemiaminals. The presence of numerous signals in the region 95–104 ppm results from a number of possible neighboring groups in the polymer chain in the vicinity of the created hemiaminal bonds. This may be also a consequence of the polydispersity of the polymer chain length that gives rise to the multiplicity of the peaks. To further characterize the material, FTIR spectra were also recorded for unmodified P[MA-co-S] and the P[MA-co-S]-AuNP@C8 nanocomposite (see Fig. S3†).
3.5 Cellulose
Cellulose is another example of a natural polysaccharide material that can covalently bind to the aminothioalkil functionalized NPs. The binding mechanism is in this case essentially the same as for starch. The modification procedure was simple, as it consisted only of immersing of a piece of cellulose paper in an aqueous solution of the AuNP@C8. The adsorption process was completed within minutes, and manifested by a change of the color of the sample from white to pink. The modified paper sample is shown in Fig. 2f.3.6 Indium tin oxide (ITO)
To further explore possible applications of the aminothioalkil functionalized NPs, we also checked their ability to adsorb on the surface of silica derivative materials, such as indium tin oxide (ITO). In this case, it is expected that the adsorption process is driven rather by electrostatic interaction of the negatively charged ITO surface and the positively charged surface of the NPs, not by the formation of covalent bonds. We successfully modified the surface of an ITO plate by immersing it in a solution of AgNPs coated with C8 ligands. The ITO plate covered with the AgNPs had clearly yellow color, as can be seen in Fig. S7.†3.7 The role of NPs in the process of bond formation
The formation of amide bonds from carboxylic acids and amines at ambient conditions is energetically unfavorable (endoergic)37 and usually possesses a high energy barrier.38The formation of amide bonds from carboxylic acids and amines at ambient conditions is energetically unfavorable (endoergic)37 and usually possesses a high energy barrier.38
The peptide bond formation is a two-stage process. An acid–base reaction occurs first, yielding a stable salt according to the equation (see also Fig. 6a):39
| R–COOH + R′–NH2 ⇄ R–COO− H3N+–R′ ⇄ R–CO–NH–R′ | (1) |
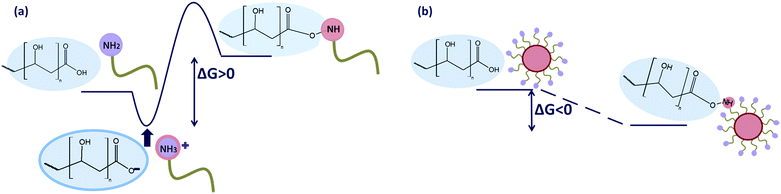 | ||
| Fig. 6 The energetic states of substrates, intermediate products and final products of peptide bond formation reaction in the absence (a) and in the presence of the NPs (b). | ||
It follows that the equilibrium of the bond formation reaction is strongly shifted in the hydrolysis direction,40 and does not occur in the opposite direction spontaneously at ambient conditions.41 To increase the amide synthesis reaction yield, activating agents or catalysts must be employed, since direct condensation of the formed salt takes place only at elevated temperatures (160–180 °C).42 There are several methods of imide bond creation described in the literature, but here, like in the case of amides, such reactions take place between amines and activated carboxyl derivatives such as carboxylic anhydrides or carboxyl acid chlorides.43 Inactivated carboxyl acids do not yield imides in reactions with amines. Acylation of amines by carboxylic esters may also occur, but the reactivity of simple esters (methyl, ethyl) in this conversion is minor, making catalyst or high pressure utilization necessary.44 Imides may also be formed in a reaction between an amide group and an adjacent ammonium carboxylic salt or carboxyl acid group. However, these reactions require high temperatures of several hundred degrees.45 The above examples clearly show that when an imide is formed at ambient conditions a reaction enhancer is necessary. We have however observed that both amide and imide bonds were created at room temperature when the amine-terminated ligand was attached to the NP surface. This follows that in such conditions the reactivity of the amine terminal group towards peptide and imide bond formation is substantially enhanced. The reaction Gibbs free energy, ΔG, is a quantity that determines the reaction direction, having a negative value for favored reactions and positive for disfavored ones. Because the enthalpy of the amide or imide formation is negative at ambient temperatures,46,47 the drop of the reaction entropy, ΔS, is responsible for the positive value of ΔG of the amide or imide formation. This is expected since the binding of the free amines to the polymer backbone considerably reduces their motion liberty (both transitional and rotational), significantly decreasing their entropy, and making the contribution TΔS negative. When the ligands are attached to the NPs the loss of entropy is significantly reduced because the ligands are pre-immobilized prior to the bond formation reaction. Most likely, in the NP-mediated bond formation the entropy contribution is substantially diminished. This makes ΔG negative, allowing the reaction to proceed spontaneously. The NPs play thus a role of the reaction enhancing agent, since it is the immobilization of the ligands on its surface that makes the reaction exoergic at ambient conditions. This role of the NPs is explained schematically in Fig. 6.
The fact that the covalent amide bonds are not formed in a reaction between free aminothioalkil molecules and PVA matrix, but are evident when the reacting ligands are attached to the surface of NPs is remarkable. It proves that the presence of the NPs is necessary for this amide or imide bond formation reaction to occur. To investigate further the bond formation, reaction of PVA polymer with free ligands was performed. The conditions of the reactions with free ligand (the concentration of the ligand, pH, the reaction time etc.) were exactly the same as in the case of the bond formation reactions involving the NPs. This allowed us to eliminate the possibility of any other factors influencing the reactions investigated. We recorded the FTIR spectra of the products of the reactions of PVA with free ligands (PVA-C8) and compared them with the corresponding FTIR spectra of PVA–AuNP@C8 nanocomposites. The FTIR spectrum of the mixture of PVA-C8 is shown in Fig. 4. Compared to the spectrum of the unmodified PVA polymer, the FTIR spectrum of PVA-C8 exhibits growth of the intensity of the right branch of the doublet signal at about 1710 cm−1, which is characteristic for protonated imines.34 The presence of imines is not surprising as they are formed spontaneously in ambient conditions. The FTIR spectrum of PVA-C8 displays also the presence of carboxylate aminium salt, evidenced by the signal at 765 cm−1 corresponding to the rocking of –NH3+ group. This salt is formed instead of amide bonds, which are not present in the PVA-C8 (see also Fig. 6a). Another evidence of the important role of the NPs in the binding reaction provided the synthesis of the P[MA-co-S]–AuNP@C8 composite. The reaction between an amine and acid anhydride functional group occurs usually in basic conditions (in the presence of triethyl amine).48
In the method employed in this work such bonds are formed in acidic conditions, which indicates that an equilibrium between protonated and neutral ligands must exist on the surface of the NPs. Even in highly acidic conditions not all ligands are in the protonated state and at least a small percentage of the ligands remains uncharged. This is consistent with recent reports49 showing dependence of ionization of ligands immobilized on the NP surface on the curvature of the NPs. It was shown that the immobilization of ionizable ligands may influence their acid–base equilibrium. The equilibrium in such conditions is shifted towards the uncharged state, compared to ligands in bulk solution, if the curvature of the NP is small.
Because it is expected that the curvature of the NP influences the equilibrium between the protonated and ambient terminal amino groups of the surface ligands, affecting the rate of the reaction between these functional groups and the polymer, an extension the method to other NPs with similar curvature, e.g., carbon NPs, would be possible. Although the principal assumptions of the method should also apply to such NPs, the functionalization technique would have to be adjusted. The thiol group does not have high affinity towards the surface of carbon NPs, therefore a different anchoring group would have to be used.
4. Conclusions
To conclude, we have developed a simple and highly reproducible ex situ strategy for polymeric nanocomposite preparation. To synthesize these materials we have functionalized noble metal NPs with novel aminothioalkil ligands. Such NPs are capable to link chemically via amide, imide, imine, or aminal bonds with a variety of polymers possessing carbonyl groups or hydroxyl groups than can be converted, upon oxidation, into carbonyl groups. An important advantage of the described method is that the binding of the NPs to the matrix occurs spontaneously at ambient conditions, and does not require any external stimuli, such as temperature or UV irradiation. The NPs are distributed uniformly within the resulting nanocomposite and do not leach when immersed in aqueous solutions. We have brought forward arguments that the binding reaction is facilitated by the presence of the NPs. The ability of the aminothioalkil coated NPs to bind covalently with a wide range of polymeric matrices offers new opportunities in the field of material engineering. In particular, the use of natural polymers, such as polysaccharides as the matrix for inorganic nanofillers is an appealing approach to synthesize functional eco-friendly metamaterials. Composites obtained in this way have an important advantage over other materials because they are easily decomposed. In our research we have demonstrated that potato starch and cellulose can be successfully employed as the polysaccharide biodegradable matrix of choice. Utilizing such biodegradable polymeric materials as the hosts for the NPs incorporation is an attractive way to overcome the negative effect of man-made materials on the environment. Also, the method uses water soluble NPs, which would be advantageous in the fabrication of the nanocomposites, since it would eliminate the need for use of large amounts of organic solvents, making the presented synthetic protocol even more environmentally friendly.Acknowledgements
This work was supported by the project operated within the Foundation for Polish Science Team Programme co-financed by the EU “European Regional Development Fund” Grant No. TEAM/2010-6/4.References
- A. Sihvola, Electromagnetic Mixing Formulas and Applications, IEEE Electromagnetic Wave Series, IEEE Pub., London, UK, 1999, vol. 47 Search PubMed.
- K. D. Min, M. Y. Kim, K. Y. Choi, J. H. Lee and S. G. Lee, Polym. Bull., 2006, 57, 101–108 CrossRef CAS.
- H. Wang, P. Xu, W. Zhong, L. Shen and Q. Du, Polym. Degrad. Stab., 2005, 87, 319–327 CrossRef CAS PubMed.
- S. J. Su and N. Kuramoto, Synth. Met., 2000, 114, 147–153 CrossRef CAS.
- J. S. Taurozzi, H. Arul, V. Z. Bosak, A. F. Burban, T. C. Voice, M. L. Bruening and V. V. Tarabara, J. Membr. Sci., 2008, 325, 58–68 CrossRef CAS PubMed.
- J. Kim and B. van der Bruggen, Environ. Pollut., 2010, 158, 2335–2349 CrossRef CAS PubMed.
- C. L. Lu and B. Yang, J. Mater. Chem., 2009, 19, 2884–2901 RSC.
- K. L. Kelly, E. Coronado, L. L. Zhao and G. C. Schatz, J. Phys. Chem. B, 2003, 107, 668–677 CrossRef CAS.
- M. A. El-Sayed, Acc. Chem. Res., 2001, 34(4), 257–264 CrossRef CAS PubMed.
- M. A. El-Sayed, Acc. Chem. Res., 2004, 37, 326–333 CrossRef CAS PubMed.
- R. Jin, S. Sun, Y. Yang, Y. Xing, D. Yu, X. Yu and S. Song, Dalton Trans., 2013, 7888–7893 RSC.
- X. Zhou, W. Xu, G. Liu, D. Panda and P. Chen, J. Am. Chem. Soc., 2010, 132, 138–146 CrossRef CAS PubMed.
- X. Li, L. Wang, Y. Fan, Q. Feng and F. Z. Cui, J. Nanomater., 2012, 2012, 548389 Search PubMed.
- L. Reijnders, Polym. Degrad. Stab., 2009, 94, 873–876 CrossRef CAS PubMed.
- A. Travan, C. Pelillo, I. Donati, E. Marsich, M. Benincasa, T. Scarpa, S. Semeraro, G. Turco, R. Gennaro and S. Paoletti, Biomacromolecules, 2009, 10, 1429–1435 CrossRef CAS PubMed.
- K. Kulthong, S. Srisung, K. Boonpavanitchakul, W. Kangwansupamonkon and R. Maniratanachote, Part. Fibre Toxicol., 2010, 7, 8 CrossRef PubMed.
- Y. J. Kim, J.-H. Kim, S. W. Ha, D. Kwon and J.-K. Lee, RSC Adv., 2014, 4, 43371–43377 RSC.
- K. Heo, C. Miesch, T. Emrick and R. C. Hayward, Nano Lett., 2013, 13, 5297–5302 CrossRef CAS PubMed.
- M. J. Lou, J. Q. Zhao, W. Tang and C. S. Pu, Appl. Surf. Sci., 2005, 249, 76–84 CrossRef PubMed.
- S. Y. Kwak, S. H. Kim and S. S. Kim, Environ. Sci. Technol., 2001, 35(11), 2388–2394 CrossRef CAS.
- Y. J. Kim, J.-H. Kim, S.-W. Ha, D. Kwon and J.-K. Lee, RSC Adv., 2014, 4, 43371–43377 RSC.
- P. L. Kuo and W. F. Chen, J. Phys. Chem. B, 2003, 107, 11267–11272 CrossRef CAS.
- B. S. Karnik, S. H. Davies, M. J. Bauman and S. J. Masten, Environ. Sci. Technol., 2005, 39(19), 7656–7661 CrossRef CAS.
- W. I. Choi, J. Y. Kim, S. U. Heo, Y. Y. Jeong, Y. H. Kim and G. Tae, J. Controlled Release, 2012, 162, 267–275 CrossRef CAS PubMed.
- I. M. Daniel, E. F. Gdoutos and Y. D. S. Rajapakse, Major Accomplishments in Composite Materials and Sandwich Structures, Springer Science & Bussiness Media, 2009 Search PubMed.
- H. Luo and D. Gersappe, Macromolecules, 2004, 37, 5792–5799 CrossRef CAS.
- A. Ghosh, C. R. Patra, P. Mukherjee, M. Sastry and R. Kumar, Microporous Mesoporous Mater., 2003, 58, 201–211 CrossRef CAS.
- P. J. Roth and P. Theato, Macromol. Chem. Phys., 2012, 213, 2550–2556 CrossRef CAS PubMed.
- G. Kickelbick, Prog. Polym. Sci., 2003, 28, 83–114 CrossRef CAS.
- M. N. Martin, J. I. Basham, P. Chando and S. K. Eah, Langmuir, 2010, 26(10), 7410–7417 CrossRef CAS PubMed.
- L. Chen, W. Zhao, Y. Jiao, X. He, J. Wang and Y. Zhang, Spectrochimica Acta Part A, 2007, 68, 484–490 CrossRef PubMed.
- K. Kulp and K. Lorenz, Cereal Chem., 1980, 58(1), 46–48 Search PubMed.
- B. Xia, Q. Cui, F. He and L. Li, Langmuir, 2012, 28, 11188–11194 CrossRef CAS PubMed.
- G. Socrates, Infrared and Raman characteristic Group Frequencies: tables and charts, John & Sons, 2004 Search PubMed.
- E. Chiellini, A. Corti, S. D'Antone and R. Solaro, Prog. Polym. Sci., 2003, 28, 963–1014 CrossRef CAS.
- J. B. Lambert, Introduction to Organic Spectroscopy, Macmillan, 1987 Search PubMed.
- M. Kurzynski, The Thermodynamic Machinery of Life, Springer Science & Business Media, 2006 Search PubMed.
- A. W. Flegmann and R. J. Tattersall, Mol. Evol., 1979, 12, 349–355 CrossRef CAS.
- C. A. G. N. Montalbetti and V. Falque, Tetrahedron, 2005, 61, 1082710852 CrossRef PubMed.
- R. V. Ulijn, B. D. Moore, A. E. M. Janssen and P. J. Halling, J. Chem. Soc., Perkin Trans. 2, 2002, 2, 1024–1028 RSC.
- E. Valeur and M. Bradley, Chem. Soc. Rev., 2009, 38, 606–631 RSC.
- B. S. Jursic and Z. Zdravkovski, Synth. Commun., 1993, 23, 2761–2770 CrossRef CAS PubMed.
- J. Zabicky, The chemistry of amides, John Wiley & Sons, London, 1970 Search PubMed.
- M. B. Smith and J. March, March’s advanced organic chemistry. Reactions, mechanism, and structure, John Wiley & Sons, Inc., 2007 Search PubMed.
- R. T. Morrison and R. N. Boyd, Chemia Organiczna, Wydawnictwo naukowe PWN, Warszawa, 2009 Search PubMed.
- I. I. Marochkin and O. V. Dorofeeva, Comput. Theor. Chem., 2012, 991, 182–191 CrossRef CAS PubMed.
- Y. R. Luo, Comprehensive Handbook of Chemical Bond Energies, CRC Press, Boca Raton, Florida, 2007 Search PubMed.
- S. B. Kalindjian, D. J. Dunstone, C. M. R. Low, M. J. Pether, S. P. Roberts, M. J. Tozer, G. F. Watt and N. P. Shankley, J. Med. Chem., 2001, 44(8), 1125–1133 CrossRef CAS PubMed.
- D. Wang, R. J. Nap, I. Lagzi, B. Kowalczyk, S. Han, B. A. Grzybowski and I. Szleifer, J. Am. Chem. Soc., 2011, 133, 2192–2197 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra12474c |
| This journal is © The Royal Society of Chemistry 2015 |