Optimization and device application potential of oxide–metal–oxide transparent electrode structure†
Yun Cheol Kima,
Su Jeong Leea,
Hanearl Jungb,
Bo-Eun Parkb,
Hyungjun Kimb,
Woong Lee*c and
Jae-Min Myoung*a
aDepartment of Materials Science and Engineering, Yonsei University, 50 Yonsei-ro, Seodaemun-gu, Seoul 120-749, Republic of Korea. E-mail: jmmyoung@yonsei.ac.kr
bSchool of Electrical and Electronic Engineering, Yonsei University, 50 Yonsei-ro, Seodaemun-gu, Seoul 120-749, Korea
cSchool of Materials Science and Engineering, Changwon National University, 20 Changwondaehak-ro, Changwon, Gyeongnam 641-774, Korea. E-mail: woonglee@changwon.ac.kr
First published on 24th July 2015
Abstract
Structural optimization of the indium zinc oxide (IZO)–Ag–IZO oxide–metal–oxide (OMO) transparent flexible electrode structure was carried out in terms of the thickness of the Ag layer based on the Haacke figure of merit. While showing sufficient sheet resistance and visible transmittance, the optimized OMO structure also exhibited good resistance to fracture under repeated bending which resulted in very small change in sheet resistance over the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of repeated bending. Low sheet resistance was found to be beneficial to lowering the contact resistances at the source and drain electrodes when the OMO structure was applied to a model thin film transistor (TFT) as revealed in the improved device performances. Device application potential of the OMO structure was demonstrated in a fully transparent TFT formed on a glass substrate in which the source, drain, and back gate electrodes were all formed using the OMO structure.
000 cycles of repeated bending. Low sheet resistance was found to be beneficial to lowering the contact resistances at the source and drain electrodes when the OMO structure was applied to a model thin film transistor (TFT) as revealed in the improved device performances. Device application potential of the OMO structure was demonstrated in a fully transparent TFT formed on a glass substrate in which the source, drain, and back gate electrodes were all formed using the OMO structure.
1 Introduction
Flexible optoelectronic devices are regarded as one of the emerging types of device. These devices are to be constructed using polymer materials for flexibility and therefore they require essentially transparent and flexible electrodes that can be processed at low temperatures. For transparent electrodes, indium tin oxide (ITO) is widely used due to its outstanding electrical and optical properties. However, if ITO films are deposited at low temperatures, their electrical properties, chemical stabilities, and mechanical properties are degraded.1 As alternatives, some candidate materials such as carbon nanotubes (CNT),2–4 metal nanowires,5–7 and conducting polymers8 have been proposed, but these materials still have shortcomings regarding performance stability and structural uniformity issues.9–14If mechanical flexibility and stability under repeated bending are primarily considered in addition to electrical conductivity, metals will be good candidates. Metals can transmit light if they are sufficiently thin and if they have low refractive index approaching zero.15 In terms of low refractive index (about 0.15 in the visible range) not to mention good electrical properties, Ag is a good candidate. Ag films can be deposited with microstructural continuity if the thickness exceeds about 10 nm.16 However, Ag films show low optical transmittance of about 47% at this thickness due to strong surface reflection. Such reflection can be reduced significantly if suitable dielectric layers are attached on the surfaces of the Ag film to modulate the surface plasmon coupling at the interfaces.17
Hence, oxide–metal–oxide (OMO) structures have been investigated as potential transparent flexible electrodes for flexible optoelectronic devices. For an oxide material in this structure, indium zinc oxide (IZO) is a good candidate. It can be deposited at low temperatures without post-annealing, which facilitates the application to flexible polymer substrates.18,19 Its amorphous structure contributes to lower film stresses, which would be beneficial to improved mechanical stability.20,21 In addition, it can be prepared with excellent surface smoothness for good electrical and optical properties.22 While some works are available on the preparation and characterization of IZO–Ag–IZO OMO electrode structures,23–25 there have been few reports on the optimization of the stacking structure including the thickness of the Ag layer and on the application of the electrodes to transparent electronic devices. This study therefore aims at the optimization of the IZO–Ag–IZO OMO electrode structure and the evaluation of the application potential of this OMO structures to the transparent electronic devices.
2 Experimental
IZO–Ag–IZO multilayer films were prepared on glass substrates at room temperature by in situ sputtering using IZO (90 wt% In2O3 + 10 wt% ZnO) and Ag (5N) targets. Initially, the 40 nm-thick top IZO layer was sputtered on the Ag layer 40 nm-thick IZO layer was sputtered as the bottom layer at the RF power of 200 W, a working pressure of 10 mTorr and an Ar flow rate of 50 sccm. Then Ag layer was continuously deposited on the bottom IZO layer with the thickness of 4 to 20 nm in steps of 2 nm at a DC power of 20 W, a working pressure of 10 mTorr and an Ar flow rate of 50 sccm. The thicknesses were controlled by varying the deposition time based on the deposition rate and then confirmed by stereoscopic ellipsometry using two different ellipsometers (SE MG-Vis 1000, Nanoview and alpha-SE, J. A. Woollam) to minimize errors. Finally, with identical deposition conditions used for the bottom electrode. The conductivity of IZO–Ag–IZO electrodes as a function of the Ag thickness were measured using a four-point probe measurement system (CMT-SR1000N, AiT) and optical properties were measured by an ultraviolet-visible spectrophotometer (UV-vis-NIR, V-670, JASCO).Mechanical stability of the IZO–Ag–IZO electrodes was evaluated by monitoring the changes in the sheet resistance with repeated bending. For the bending test, the samples were bent in the convex form with the radius of curvature of 2 mm by applying an in-plane compressive load on the two opposite edges at the ramp rate of 3.67 mm s−1 using a bending machine (Flexible Materials Tester, Hansung Systems Inc.). One the sample was bent it was held for 1 s and then the load was released.
Model devices for the feasibility study were prepared in the following manner. A 30 nm-thick amorphous In–Ga–Zn–O (a-IGZO) channel layer was deposited by sputtering on the SiO2/p++-Si substrate where the 300 nm-thick SiO2 dielectric layer was formed by thermal oxidation. The DC power, working pressure and Ar/O2 mixture ratio were maintained at 150 W, 5 mTorr and 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. Subsequently, the samples were thermally annealed at 300 °C for 1 h in air. OMO source and drain electrodes were attached in one device while single layer electrodes were attached in the control device. The performance characteristics of these a-IGZO devices were analyzed by a semiconductor parameter analyzer system (Agilent B1500A, Agilent Technologies).
1, respectively. Subsequently, the samples were thermally annealed at 300 °C for 1 h in air. OMO source and drain electrodes were attached in one device while single layer electrodes were attached in the control device. The performance characteristics of these a-IGZO devices were analyzed by a semiconductor parameter analyzer system (Agilent B1500A, Agilent Technologies).
A fully transparent a-IGZO thin film transistor (TFT) was prepared to demonstrate the applicability of the OMO electrodes. First, OMO structure was deposited as the bottom gate electrode on a glass substrate, which was followed by the deposition of 30 nm-thick HfO2 gate dielectric by a pulsed plasma-enhanced chemical vapor deposition (PECVD) at 200 °C using tris(dimethylamino)cyclopentadienyl hydrogen fluoride (TDMA-HF) and O2 as the precursor for hafnium and oxygen. Next, 30 nm-thick a-IGZO channel layer was deposited on the HfO2 gate dielectric layer. Finally, OMO source and drain electrodes were attached to the channel to finish device preparation.
3 Results and discussion
In the OMO electrode structure, most of the electrical transportation is born by the metallic interlayer. On the other hand, the metallic layer should be kept as thin as possible to ensure sufficient optical transmittance. Hence, it was first investigated how the sheet resistance of the OMO structure changed with varying thickness of the Ag interlayer. Concerning the IZO top and bottom layers, their major role is the maximization of the light transmission via the modulation of surface plasmon coupling. It has been reported that the best light transmittance can be achieved when their thicknesses are between 30 and 50 nm.26 Therefore, the thickness of IZO layers were fixed at 40 nm. Fig. 1(a) shows that the sheet resistance of the OMO film decreased rapidly to 17.7 Ω sq−1 as the thickness of Ag layer increased from 4 to 6 nm and then decreased slowly to 7.76 Ω sq−1 up to the thickness of 10 nm. Further increasing the Ag layer thickness beyond 10 nm only resulted in marginal decrease of the sheet resistance. For comparison purpose, sheet resistances of the single Ag layer and Ag–IZO bi-layer films were also measured and similar trends were observed as shown in Fig. 1(a).In Fig. 1(a), it is noticed that as the Ag layer thickness exceeds 10 nm, the sheet resistances of the three electrode structures are almost identical indicating that the electrical conduction is mainly through the Ag layer at these thicknesses. It should also be noted that the sheet resistance of the 4 nm-thick single Ag layer could not be measured, which suggests that the electrical conduction of the OMO and Ag–IZO bi-layer films are due to the IZO layer(s) only. It is known that the Ag layer becomes structurally continuous when its thickness is larger than about 10 nm.16 In other words, Ag layer thinner than 10 nm may contain some structural defects such as discontinuities. Observations of the morphological evolution of the Ag layer with increasing thickness shown in the ESI (Fig. S1†) revealed that the Ag layer evolved from isolated islands (at the thickness of 4 nm) to continuous film (at 10 nm) through the coalescence of the islands forming trenched and voided films (at 6 and 8 nm, respectively). Such a morphological evolution process is well correlated to the changes in the sheet resistances with increasing thickness of the Ag layer.
For transparent electrode applications, visible transmittance is also of primary concern in addition to the electrical properties. Fig. 1(b) shows the changes in the transmittance of the OMO electrode structure with varying Ag layer thickness. When the Ag layer was 4 nm thick, the optical transmittance at 550 nm wavelength was 78.2%. As the thickness of the Ag layer increased, the transmittance at 550 nm increased gradually to 96.3% at the thickness of 12 nm and then decreased with further increasing Ag layer thickness. Initial increase in the visible transmittance at 550 nm wavelength with increasing Ag layer thickness seems to be related to the morphological evolution of the Ag layer from the isolated islands to the continuous film. If the Ag layer is not structurally uniform, it is expected that its interface with the IZO layers are uneven, which then results in the scattering of light at the interface reducing the transmittance.26 Only when the Ag layer becomes structurally uniform and continuous, smooth and well-defined interface with the IZO layer may be expected as shown in the cross-sectional composition profile and the transmission electron microscopy contrast image (ESI, Fig. S2†). Once the continuous film is formed, increasing the thickness is not beneficial to the transmittance since metal sheets become rapidly opaque to the electromagnetic waves with increasing thickness.
Effects of the thickness of Ag mid-layer on the electrical and optical properties of the OMO electrode can be summarized as follows. First, sheet resistance decreases with increasing thickness of Ag layer. However, after the structurally continuous layer is formed, the thickness has marginal effect on the sheet resistance decrease. Second, optical transmittance reaches a maximum when the Ag layer thickness somewhat exceeds the minimum thickness to form the structural continuity and then decreases. Based on these observations, it is now necessary to establish the optimal thickness of the Ag layer in terms of both the electrical and optical properties. For this purpose, figure of merit (FOM) was evaluated for a 10 × 10 cm2 electrode using the following equation as defined by Haacke:27
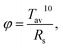 | (1) |
As the OMO structure was developed for flexible transparent electrode, it is necessary to investigate whether the IZO–Ag–IZO OMO electrode considered in this study can withstand repeated bending. Fig. 3(a) shows the changes in the sheet resistance of the optimized OMO electrode with repeated bending cycle with the radius of curvature of 2 mm, as % increases in the sheet resistance with respect to the initial resistance. Also shown are the changes in the sheet resistance of a single IZO electrode having the same thickness. In the case of the OMO electrode, there was virtually no increase in the sheet resistance (from Ri = 5.65 to Rf = 5.86 Ω sq−1) after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles considering the measurement errors. On the other hand, single layer IZO electrode having equal thickness showed substantial increase in the sheet resistance even after 10 cycles of bending. After 1000 cycles, the sheet resistance increased by 1146%.
000 cycles considering the measurement errors. On the other hand, single layer IZO electrode having equal thickness showed substantial increase in the sheet resistance even after 10 cycles of bending. After 1000 cycles, the sheet resistance increased by 1146%.
Such a stability of the OMO structure in terms of the sheet resistance under repeated bending is attributed to the presence of the Ag mid-layer.28,29 On bending, it is possible that the crack initiated on the tensile side (convex outer surface) of the IZO layer and this crack can grow under repeated bending.30 However, the Ag layer, which is highly resistant to fracture, can function as a crack stopper as in the case of the fiber-reinforced or laminated composite materials.29 This way, growth of surface cracks on the IZO layer can be retarded while the Ag conduction layer itself, which is responsible for most of the electric conduction, is left intact under cyclic loading. Contrary to this, if the electrode is formed by a single IZO layer, which is relatively brittle compared with the OMO multilayer electrode, crack can propagate with little resistance once it is formed. Consequently, cracked single layer IZO electrode will show very high electrical resistance. Indeed, as shown in Fig. 3(b) and (c), only small number or relatively short cracks were observed on the surface of the OMO electrode after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of bending whereas the surface of the single layer IZO electrode after only 1000 cycles of bending was characterized by number of long cracks. These results demonstrate much superior mechanical stability of the OMO structure over single layer IZO structure as a flexible electrode.
000 cycles of bending whereas the surface of the single layer IZO electrode after only 1000 cycles of bending was characterized by number of long cracks. These results demonstrate much superior mechanical stability of the OMO structure over single layer IZO structure as a flexible electrode.
Application potential of the IZO–Ag–IZO OMO electrode has been evaluated through the application of this electrode to a model thin film transistor (TFT) having an a-IGZO channel and an SiO2 gate dielectric formed on a p++-Si back gate. In this device, OMO source/drain (S/D) electrodes were defined on the a-IGZO active region. As a control, another a-IGZO TFT was prepared using single layer IZO for the S/D electrodes. Fig. 4(a) and (b) show the output characteristics of the two devices. It can be seen that the drain–source currents, IDS, were much higher in the TFT with OMO S/D electrodes than in the control device with IZO counterparts, for a given gate voltage, VG. Consequently, superior performance of the device with the OMO S/D electrodes was obtained as evident in the transfer characteristics compared in Fig. 4(c) and (d). The device performances were estimated using these transfer characteristics and are summarized in Table 1. It can be seen in Table 1 that the a-IGZO TFT with the OMO S/D electrodes showed higher field effect mobility, μe, higher ON/OFF ratio, Ion/Ioff, lower threshold voltage close to 0, VT, and lower subthreshold swing, S.
| μe (cm2 V−1 s−1) | Ion/Ioff | VT (V) | S (V per decade) | |
|---|---|---|---|---|
| OMO S/D TFT | 11.87 | 5.87 × 107 | 0.73 | 0.73 |
| IZO S/D TFT | 8.19 | 5.13 × 107 | 7.92 | 1.01 |
These improved performances are attributed to the decreased contact resistance at the S/D electrodes, RSD. Using RSD, μe can be expressed as31
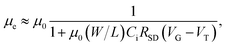 | (2) |
| IDS = μe(W/L)Ci(VG − VT)(VDS − 2RSDIDS), | (3) |
The higher IDS for a given VG will decrease the S, since S is defined as
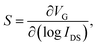 | (4) |
Finally, lower contact resistance at the S/D electrodes means the lower resistance to the carrier injection and collection at the S/D electrodes, which will then increase effectively the carrier concentration in the channel at ON state. This is then linked to the negative shift of the VT from 7.92 to 0.73 V (Table 1).
Having deduced that the improved device performances can be attributed to the lower contract resistance at the S/D electrodes due to the adoption of the OMO film, it is necessary to show whether the contract resistance was indeed lowered. Hence, the contact resistance RSD was evaluated by the transmission line measurement (TLM). The TLM patterns consisted of the channel width of 400 μm and the distributed channel lengths of 100, 150, 200, 250, 300, and 350 μm, respectively. The contact resistance of the IZO–Ag–IZO OMO electrodes and the single layer IZO electrodes on the a-IGZO active layer are compared in Fig. 5 for different gate voltages ranging from 5 to 20 V with 5 V intervals. It is seen that while the RSD of the OMO electrodes changed from 22.4 to 4.8 MΩ with increasing VG, those of the IZO electrodes changed from 45.5 to 25.9 MΩ. Obviously, the OMO electrodes showed much lower contact resistance, which would be originated from the lower sheet resistance.
 | ||
| Fig. 5 Contact resistances of the IZO–Ag–IZO S/D electrodes and the single layer IZO S/D electrodes for various gate voltages. | ||
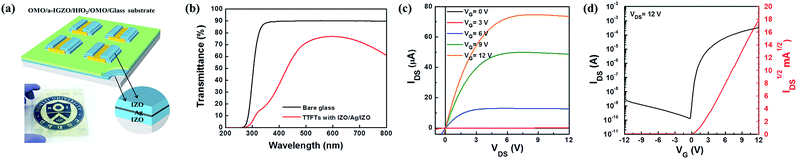
In order to further demonstrate the application potential of the OMO electrode in this study, a fully transparent bottom gate a-IGZO channel TFT was prepared on a glass substrate applying the OMO structure to the S/D and the gate electrodes. The device was fully transparent as shown in Fig. 6(a) together with the schematic illustration of the device structure. The device had the average transmittance in the visible range (380 to 750 nm) of 74.7% while the transmittance at 550 nm wavelength was 84.1% as shown in Fig. 6(b). The output and transfer characteristics shown in Fig. 6(c) and (d), respectively, indicate that the transparent TFT functioned properly with the μe, Ion/Ioff, VT, and S of 3.24 cm2 V−1 s−1, 9.81 × 108, 1.57 V and 0.16 V per decade, respectively, implying that fully transparent TFTs with all OMO electrodes can be materialized.
4 Conclusions
It was suggested through the structural optimization based on the Haacke figure of merit suggested that the IZO–Ag–IZO OMO transparent flexible electrode can have the best combination of sheet resistance (5.65 Ω sq−1) and average visible transmittance (87.7%) when the thickness of Ag mid-layer is 12 nm. This optimized OMO electrode demonstrated very good durability under repeated bending in that it showed practically no change in sheet resistance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of bending with the radius of curvature of 2 mm. When the optimized OMO structure was applied as the source and drain electrodes of an a-IGZO channel TFT, the device showed much improved performance over the control device having single layer IZO source and drain electrodes. Such improved device performance was attributed to the reduced contact resistance at the source and drain due to the enhanced electrical property of the OMO electrode. When the OMO structure was applied to the gate, source, and drain electrodes of an a-IGZO channel device, a properly functioning fully transparent thin film transistor was obtained.
000 cycles of bending with the radius of curvature of 2 mm. When the optimized OMO structure was applied as the source and drain electrodes of an a-IGZO channel TFT, the device showed much improved performance over the control device having single layer IZO source and drain electrodes. Such improved device performance was attributed to the reduced contact resistance at the source and drain due to the enhanced electrical property of the OMO electrode. When the OMO structure was applied to the gate, source, and drain electrodes of an a-IGZO channel device, a properly functioning fully transparent thin film transistor was obtained.
Acknowledgements
This work was supported by the IT R&D program of MOTIE/KEIT (No. 10042414, Development of 8th Generation (2200 × 2500 mm2) Major Equipment for Transparent Flexible Display in Large Area).Notes and references
- V. Sivaji Reddy, K. Das, A. Dhar and S. K. Ray, Semicond. Sci. Technol., 2006, 21, 1747 CrossRef
.
- Z. Wu, Z. Chen, X. Du, J. M. Logan, J. Sippel, M. Nikolou, K. Kamaras, J. R. Reynolds, D. B. Tanner, A. F. Hebard and A. G. Rinzler, Science, 2004, 305, 1273 CrossRef CAS PubMed
.
- Q. Cao, Z. T. Zhu, M. G. Lemaitre, M. G. Xia, M. Shim and J. A. Rogers, Appl. Phys. Lett., 2006, 88, 113511 CrossRef
.
- D. J. Lipomi, M. Vosgueritchian, B. C.-K. Tee, S. L. Hellstrom, J. A. Lee, C. H. Fox and Z. Bao, Nat. Nanotechnol., 2011, 6, 788 CrossRef CAS PubMed
.
- S. De, T. M. Higgins, P. E. Lyons, E. M. Doherty, P. N. Nirmalraj, W. J. Blau, J. J. Boland and J. N. Coleman, ACS Nano, 2009, 3, 1767 CrossRef CAS PubMed
.
- J. van de Groep, P. Spinelli and A. Polman, Nano Lett., 2012, 12, 3138 CrossRef CAS PubMed
.
- V. Scardaci, R. Coull, P. E. Lyons, D. Rickard and J. N. Coleman, Small, 2011, 7, 2621 CrossRef CAS PubMed
.
- J. Zou, H. L. Yip, S. K. Hau and A. K. Y. Jen, Appl. Phys. Lett., 2010, 96, 203301 CrossRef
.
- L. B. Hu, G. Gruner, J. Gong, C. J. Kim and B. Hornbostel, Appl. Phys. Lett., 2007, 90, 093124 CrossRef
.
- D. Hecht, L. Hu and G. Gruner, Appl. Phys. Lett., 2006, 89, 133122 CrossRef
.
- Y. Wu, J. Xiang, C. Yang, W. Lu and C. M. Lieber, Nature, 2004, 430, 61 CrossRef CAS PubMed
.
- Y. Long, Z. Chen, X. Zhang, J. Zhang and Z. Liu, Appl. Phys. Lett., 2004, 85, 1796 CrossRef CAS
.
- L. Hu, H. S. Kim, J.-Y. Lee, P. Peumans and Y. Cui, ACS Nano, 2010, 4, 2955 CrossRef CAS PubMed
.
- Y. H. Kim, C. Sachse, M. L. Machala, C. May, L. Müller-Meskamp and K. Leo, Adv. Funct. Mater., 2011, 21, 1076 CrossRef CAS
.
- B. O'Connor, C. Haughn, K.-H. An, K. P. Pipe and M. Shtein, Appl. Phys. Lett., 2008, 93, 223304 CrossRef
.
- S. Kundu, S. Hazra, S. Banerjee, M. K. Sanyal, S. K. Mandal, S. Chaudhuri and A. K. Pal, J. Phys. D: Appl. Phys., 1998, 31, 73 CrossRef
.
- K. Hong, J. H. Son, S. J. Kim, B. H. Koo and J. L. Lee, Chem. Commun., 2012, 48, 10606 RSC
.
- W. Lim, Y.-L. Wang, F. Ren, D. P. Norton, I. I. Kravchenko, J. M. Zavada and S. J. Pearton, Appl. Surf. Sci., 2008, 254, 2878 CrossRef CAS
.
- Y.-L. Wang, F. Ren, W. Lim, D. P. Norton, S. J. Pearton, I. I. Kravchenko and J. M. Zavada, Appl. Phys. Lett., 2007, 90, 232103 CrossRef
.
- J.-W. Kang, W.-I. Jeong, J.-J. Kim, H.-K. Kim, D.-G. Kim and G.-H. Lee, ECS Solid State Lett., 2007, 10, 75 CrossRef
.
- A. Sugimoto, H. Ochi, S. Fujimura, A. Yoshida, T. Miyadera and M. Tsuchida, IEEE J. Sel. Top. Quantum Electron., 2004, 10, 107 CrossRef CAS
.
- A. Wang, J. Dai, J. Cheng, M. P. Chudzik, T. J. Marks, R. P. H. Chang and C. R. Kannewurf, Appl. Phys. Lett., 1998, 73, 327 CrossRef CAS
.
- Y.-S. Park, H.-K. Kim and S.-W. Kim, J. Electrochem. Soc., 2010, 157, 301 CrossRef
.
- S. Boscarino, I. Crupi, S. Mirabella, F. Simone and A. Terrasi, Appl. Phys. A, 2014, 116, 1287 CrossRef CAS
.
- A. Dhar and T. L. Alford, ECS Solid State Lett., 2014, 11, 33 CrossRef
.
- K. Koike and S. Fukuda, J. Vac. Sci. Technol., 2008, 26, 444 CrossRef CAS
.
- G. Haacke, J. Appl. Phys., 1976, 47, 4086 CrossRef CAS
.
- J. Lewis, S. Grego, B. Chalamala, E. Vick and D. Temple, Appl. Phys. Lett., 2004, 85, 3450 CrossRef CAS
.
- D. P. Wang, F. Y. Biga, A. Zaslavsky and G. P. Crawford, J. Appl. Phys., 2005, 98, 086107 CrossRef
.
- S.-W. Cho, J.-A. Jeong, J.-H. Bae, J.-M. Moon, K.-H. Choi, S. W. Jeong, N.-J. Park, J.-J. Kim, S. H. Lee, J.-W. Kang, M.-S. Yi and H.-K. Kim, Thin Solid Films, 2008, 516, 7881 CrossRef CAS
.
- J. S. Lee, S. Chang, H. Bouzid, S. M. Koo and S. Y. Lee, Phys. Status Solidi A, 2010, 207, 1694 CrossRef CAS
.
- K.-H. Choi and H.-K. Kim, Appl. Phys. Lett., 2013, 102, 052103 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1 SEM micrographs showing the morphological evolution of the Ag mid-layer with increasing thickness. Fig. S2 cross-sectional structure of the optimized IZO–Ag–IZO electrode as identified by XPS depth profile, cross-sectional TEM and HRTEM image. See DOI: 10.1039/c5ra12473e |
| This journal is © The Royal Society of Chemistry 2015 |