Review on the extraction, characterization and application of soybean polysaccharide
Abstract
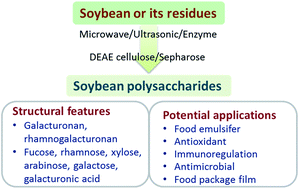
Soybean polysaccharide (SPS) is a class of soluble polysaccharide derived from soybean cotyledon, soybean meal or okara, and has broadly been used in the food industry. In recent decades, due to its attractive physicochemical properties, SPS has been developed into various emulsifiers or stabilizers for beverages. Additionally, studies have emerged to reveal its potential in biomaterial and biological applications. In this review, we critically appraise the latest literature on the extraction and the structural features of SPS, and provide a perspective on the biological applications of SPS. We focus on the current strategies for the extraction of this unique polysaccharide, specific structural features, and functional utilization of SPS. Notably, SPS-based food additives have been demonstrated to add value in biological applications due to their anticancer and immunoregulatory effects, encouraging us to use SPS directly in the area of biomedicine. Lastly, we suggest some potential directions for the development of SPS for extensive utilization in biomedicine.

 Please wait while we load your content...
Please wait while we load your content...