Facile and green aerosol-assisted synthesis of zeolites†
Abstract
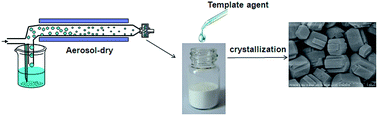
Here we report a facile and green aerosol-assisted method to synthesize zeolites. Compared with the conventional hydrothermal route, the new method is simpler and less polluting, and requires lower template amount, reaction volume, and crystallization temperature.

 Please wait while we load your content...
Please wait while we load your content...