One-step and low-temperature synthesis of carbon nanotubes with no post treatment and high purity†
Jun Wang*,
Long Zhang‡,
You song Liu and
Xiangli Guo‡
Institute of Chemical Materials, China Academy of Engineering Physics, No. 64, Mianshan Road, Mianyang City, China. E-mail: wjun927@caep.cn; Tel: +86 8162485072
First published on 10th September 2015
Abstract
A new strategy for the synthesis of carbon nanotubes without any catalyst via the reaction between difluorocarbene (CF2) radicals generated from a precursor (hexafluoropropylene oxide) and porous silicon nanowire arrays at low temperature is reported in this study. No further post treatment is needed and a high CNT purity of ∼90% is achieved. A four-step formation mechanism is proposed to understand the growth process of the carbon nanotubes.
Carbon nanotubes (CNTs), a new class of carbon-based nanomaterials consisting of sp2-hybridized atoms, have stimulated wide interests in both the experimental and theoretical scientific community due to their high thermal and electrical conductivity, as well as chemical stability.1–3 The synthesis of carbon nanotubes has always been one of the main challenges for scientific research and industrial applications.4–6 To solve this problem, diverse fabrication methods for carbon nanotubes have been studied,7–10 which indicate that the synthesis method and carbon feedstock are the two most important factors in the preparation of carbon nanotubes.11–13 Chemical vapor deposition (CVD) is the most common method used for the CNT's production. Typically, a wide variety of hydrocarbons (e.g., CH4, C2H2, C2H4) are used as carbon feedstock in the synthesis process.14 According to growth process of CNTs, the hydrocarbon precursors are decomposed to produce carbon atoms and then form CNTs. Moreover, high temperatures and catalysts are always required to improve the reactivity of carbon feedstock, and form the special nanotube structure, as well as to maximize the yield and purity. For example, many kinds of metals (typically Fe, Co, Mo or Ni) are introduced as catalysts during the synthesis process.14 However, the introduced metal catalysts are covered inside the CNTs and very hard to remove, which is bad for the purity and properties of CNTs. Therefore, preparing CNTs under low temperature process is essential to achieve high purity and good properties which are necessary for the practical applications. The synthesis of CNTs remains a key field in CNTs science and technology and plays the most important role for their wide application. Moreover, CNTs exhibit potential applications in nanocontainers and nano-reactors.6 Therefore, development new approach, which is fully compatible with microelectromechanical systems (MEMS) technology is important.
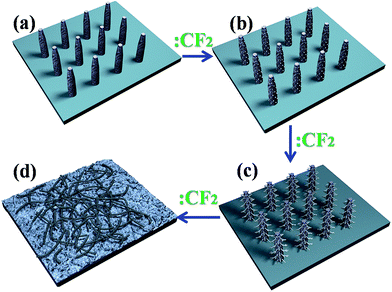
In this manuscript, we report a novel approach for low temperature and one-step synthesis of the CNTs with no post treatment and high purity by using CF2 radicals as carbon feedstacks to react with porous silicon nanowire. Fig. 1 shows the schematic of the CF2 radicals and porous silicon nanowires used for the preparation of CNTs. The hexafluoropropylene oxide (HFPO) is decomposed to form CF2 radicals at 180 °C.15 The CF2 radicals make contact and react with porous silicon nanowire to produce carbon atoms. Then, active carbon atoms assemble to form CNTs.
The porous silicon nanowire arrays were synthesized according to the method reported in literature16 (see ESI†). The nanowire array structure is characterized by SEM and TEM microscopy. The typical sample of nanowire arrays on silicon can be observed clearly by SEM images (Fig. 2a and b). The Si nanowires are covered densely the silicon substrate and exhibit uniform dimensional size with the diameter of ∼200 nm and the length up to around14,15 μm. TEM and HRTEM images show that the nanowire has porous nanostructure. The surface area of the porous silicon nanowires (scrapped off silicon substrate) is 22.3–24.6 m2 g−1. The average size and shape of the pores are not uniform, with the diameter mostly around 22.6 nm. Those porous nanostructure plays an important role in formation of CNTs.
The TEM images of the CNTs are shown in 2d–f, in which the product exhibits multi-walled structure of nanotubes with the outer diameters of CNT is 10–14 nm and inner diameters is 5–7 nm, respectively. The well-graphitized structure can be also observed form the images. Fig. 1S† shows the Raman spectrum of CNTs in the range of 500–2000 cm−1, the peak at around 1615 cm−1 (G-band) corresponding to an E2g mode of hexagonal graphite is related to the vibration of sp2− hybridized carbon atoms in a graphite layer. The D-band at about 1370 cm−1 can be ascribed to the vibration of carbon atoms with dangling bonds in the plane terminations of disordered graphite in CNTs. From the TGA test (Fig. S2†), we can see that about 90 wt% of the product is lost when we annealed the sample in air, which indicates that the carbon content in the as-prepared product is 90 wt%. Those results imply that our method can realize the synthesis of CNT with high purity and no post treatment is need.
We found that porous silicon nanowires play significant roles in the formation of CNTs. Mesoporous silica was also used as substrate and tested since it might play a templating role in guiding the initial nanotube growth. For example, Li and co-workers demonstrated aligned CNT growth on bulk mesoporous silica 17 and films 18 with iron nanoparticles embedded in the mesopores. Similarly, Zheng et al.19 successfully prepared well-aligned CNTs which were grown on mesoporous silica films by chemical vapor deposition (CVD). The template function of mesoporous silica depends on the pore structure and how the transition metals are distributed in the mesopores. In our method, porous silicon nanowires play two important roles in the preparation of CNTs. First, silicon nanowires reacted with CF2 radicals to produce carbon atoms, which is essential for the synthesis of CNTs. Second, porous silicon nanowire consisting of silicon nanoparticles play a templating role in guiding the initial nanotube growth. In order to illustrate the templating role of porous silicon nanowire, additional experimental evidence is performed. Silicon nanowire without pores and nano-silicon particles are used for synthesis of CNTs (Fig. S2†). The results indicate that no CNTs are prepared using silicon nanowire and nano-silicon particles. According to vapor–liquid–solid mechanism,20 Firstly, gas carbon feedstock (hydrocarbon) adsorbed on the surface of the catalyst particle and decomposed to form carbon atoms. Secondly, the carbon atoms dissolve and diffuse within the particle. Finally, solid carbon precipitates at the rear side of the nanoparticles to form CNTs. In this experiment, porous silicon nanowires consisting of silicon nanoparticles play a role as catalyst particle, offering growing a point for carbon atoms. To be specific, the silica on the surface of the porous silicon plays this role. The TEM image of the nanotubes also further supports the function of porous silicon (Fig. S3†). Based on the research related to the CNTs, we consider that the porous silicon nanowire is a prerequisite for the achievement of CNTs.
The representative schematic mechanism is illustrated in Fig. 3. The precursor gas HFPO can be decomposed under low temperature of 150 °C to form difluorocarbene (CF2) radicals. Then the CF2 radicals adsorb on the surface of porous silicon nanowires and react with silicon to form SiF4 gas and active carbon atoms with liberation of heat. Then, carbon atoms diffuse with SiF4 gas due to reaction heat. Subsequently, the solid carbon precipitates at the rear side of the silicon nanoparticles to form a CNT via a strong and stable sp2 bond. The porous silicon nanowires are completely consumed as the reaction progresses (Fig. S5(a)†). If excess CF2 continuously flows on the silicon surface and reacts with silicon, the graphene would be produced (Fig. S5(b) and (c)†). The HRTEM imaging (Fig. S5(d)†) clearly shows the graphene structure and d-spacing is about 0.35 nm. The overall chemical reactions involved in the thermal synthesis can be expressed with the following formulae:
| CF3CFOCF2 → :CF2 + CF3CFO↑, temp: 150 °C |
| Si + :CF2 → C + SiF4↑, ΔH = −5500 kJ kg−1. |
Compared with catalytic chemical vapour deposition (CCVD), the growth mechanism presented here is massively supported as the vapor–liquid–solid mechanism but with several significant differences. The carbon atoms are obtained by reaction between CF2 and porous silicon nanowires. The carbon atoms diffuse following the role of SiF4 gas and thermal expansion effect because of the large reaction heat (5500 kJ kg−1) in this process. Furthermore, porous silicon nanowires have two effects of reacting with carbon feedstock (CF2) to form carbon atoms and offering growing points for carbon atoms. Addition, a remarkable advantage of the strategy is that it can be done at a low reaction temperature with no catalyst and post treatment needed.
In the case of a gaseous carbon source, the CNTs growth strongly depends on the reactivity and concentration of produced CF2 free radicals from precursor decomposition. The concentration of CF2 free radicals is controlled by the precursor gas HFPO with mass flow controllers. The results suggest that the diameter of the products increase with the increased gas flow rate of the precursor. The significantly increased reaction heat results a remarkable increase in the diffusion rate of carbon atoms and thermal expansion effect.
Overall, three significant advantages are displayed in this new synthesis strategy. First, the products of all reactions (CF3CFO and SiF4) are gaseous, which is beneficial for achieving CNTs with high purity. Second, compared with CCVD synthesis, low reaction temperature (150 °C) is very convenient for integrating CNTs on silicon-based microelectromechanical systems to achieve functional nanodevices. Third, synthesis process without a catalyst would eliminate the purifying process and hold the performance of CNTs in applications. More importantly, synthesis processes without a catalyst reduce factors that interfere with the CNTs growth process and structure, which is helpful for scientists to better understand the nucleation and growth mechanism.
In conclusion, we report a new strategy to synthesis CNTs using free radical reaction between difluorocarbene(CF2) and porous silicon nanowires arrays at low temperature (150 °C) without any catalyst. The porous silicon nanowire arrays play two major roles in the preparation of CNTs as a reactant and template for carbon atoms growth. A four steps growth mechanism of CNT is proposed to understand the formation process. Moreover, the CNTs are fabricated directly on a silicon substrate, which is very convenient for integrating CNTs with silicon-based microelectromechanical systems to achieve functional micro or nano devices.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 11272292 and 11372288), Development Foundation of CAEP (No. 2014B0302041), and Young Talent Foundation of Institute of Chemical Materials (KJCX-201405,KJXZ-201403).Notes and references
- K. Kim, Y. Oh and M. F. Islam, Nat. Nanotechnol., 2012, 118, 1–5 Search PubMed; K. Gong, F. Du, Z. Xia, M. Durstock and L. Dai, Science, 2009, 323, 760–763 CrossRef CAS PubMed.
- T. W. Ebbesen and P. M. Ajayan, Nature, 1992, 358, 220 CrossRef CAS PubMed.
- K. Moothi, S. E. Iyuke, M. Meyyappan and R. Falcon, Carbon, 2012, 50, 2679–2690 CrossRef CAS PubMed; P. Vinten, P. Marshall, J. Lefebvre and P. Finnie, J. Phys. Chem. C, 2013, 117, 3527–3536 Search PubMed.
- L. T. Scott, E. A. Jackson and Q. Zhang, J. Am. Chem. Soc., 2012, 134, 107–110 CrossRef CAS PubMed.
- R. Xiang, B. Hou, E. Einarsson and P. Zhao, ASC Nano, 2013, 7, 3095–3103 CrossRef CAS PubMed; D. Tang, C. Liu, W. Yu, Y. Bando, D. Golberg and H. Cheng, ACS Nano, 2014, 8, 292–301 CrossRef PubMed.
- B. Liu, J. Liu, X. Tu, J. Zhang, M. Zheng and C. Zhou, Nano Lett., 2013, 13, 4416–4421 CrossRef CAS PubMed.
- Y. Yu, J. Chen, Z. M. Zhou and Y. D. Zhao, Dalton Trans., 2013, 42, 15280–15284 RSC.
- M. Ahmad, J. V. Anguita, V. Stolojan, J. D. Carey and S. R. P. Silva, ACS Appl. Mater. Interfaces, 2013, 5, 3861–3866 CAS.
- Y. Jia, P. Y. Wu, F. Fang, S. S. Zhou and D. Y. Peng, Solid State Sci., 2013, 18, 71–77 CrossRef CAS PubMed; P. Vinten, P. Marshall, J. Lefebvre and P. Finnie, J. Phys. Chem. C, 2013, 117, 3527–3536 Search PubMed.
- H. Li, L. Guan, Z. Shi and Z. Gu, J. Phys. Chem. B, 2004, 108, 4573–4578 CrossRef CAS.
- I. V. Anoshkin, A. G. Nasibulin and H. Jiang, Carbon, 2014, 78, 130–135 CrossRef CAS PubMed.
- H. Dai, Acc. Chem. Res., 2002, 35, 1035–1044 CrossRef CAS PubMed.
- J. R. Valencia and T. Diene, Nature, 2014, 512, 61–64 CrossRef PubMed.
- J. Prasek, J. Drbohlavov and R. Kizek, J. Mater. Chem., 2011, 21, 15872 RSC.
- J. Wang, X. Song, R. li, J. Shen and G. Yang, Appl. Surf. Sci., 2012, 258, 9782–9785 CrossRef CAS PubMed.
- K. Shi, J. Yan, E. Lester and T. Wu, Ind. Eng. Chem. Res., 2014, 53, 15012–15019 CrossRef CAS.
- W. Z. Li, S. S. Xie, L. X. Qian, B. H. Chang, B. S. Zou, W. Y. Zhou and G. Wang, Science, 1996, 274, 1701 CrossRef CAS.
- S. Xie, W. Li, Z. Pan, B. Chang and L. Sun, J. Phys. Chem. Solids, 2000, 61, 1153 CrossRef CAS.
- F. Zheng, L. Liang and C. L. Aardahl, Nano Lett., 2002, 2, 729–732 CrossRef CAS.
- J. P. Tessonnier and D. S. Su, ChemSusChem, 2011, 4, 824–847 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis process of Si nanowires, TAG curve of CNTs, TEM images of silicon nanoparticles and nanowires, FESEM image of silicon substrate after reaction and TEM images of CNT and carbon nanofilm. See DOI: 10.1039/c5ra12365h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |