Microphase separated sepiolite-based nanocomposite blends of fully sulfonated poly(ether ketone)/non-sulfonated poly(ether sulfone) as proton exchange membranes from dual electrospun mats
Abstract
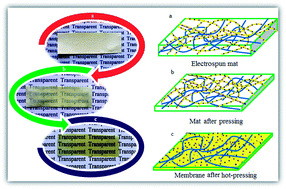
In this study, nanocomposite blends of fully sulfonated poly(ether ketone) (PEK) and non-sulfonated poly(ether sulfone) (PES) were prepared from a dual electrospinning process. Sepiolite was used as the nanoparticle and was dispersed only in the non-sulfonated PES matrix to avoid the barrier effects of nanoparticles against protons in these proton exchange membranes. As a novel and special technique, using this dual electrospinning process and melting only in the non-sulfonated sepiolite dispersed fibers resulted in membranes in which ionic paths (fibers of fully sulfonated PEK) were embedded in a sepiolite dispersed non-ionic matrix. The presence of these ionic channels was proved by transmittance electron microscopy (TEM) with staining. The thermal and mechanical properties and the water absorption, dimensional stability, and proton conductivity of these hydrophilic/hydrophobic phase separated membranes were measured and investigated for samples with different sepiolite and sulfonated fiber contents.

 Please wait while we load your content...
Please wait while we load your content...