Extractive desulfurization of dibenzothiophene by a mixed extractant of N,N-dimethylacetamide, N,N-dimethylformamide and tetramethylene sulfone: optimization by Box–Behnken design
Kun Zhaoab,
Yan Chengab,
Hongyu Liu*ab,
Chunping Yang*abc,
Lu Qiuab,
Guangming Zengab and
Huijun Heab
aCollege of Environmental Science and Engineering, Hunan University, Changsha, Hunan 410082, P.R. China. E-mail: hyliu@hnu.cn; yangc@hnu.edu.cn; Tel: +86 731 88823987
bKey Laboratory of Environmental Biology and Pollution Control (Hunan University), Ministry of Education, Changsha, Hunan 410082, P. R. China
cZhejiang Provincial Key Laboratory of Solid Waste Treatment and Recycling, College of Environmental Science and Engineering, Zhejiang Gongshang University, Hangzhou, Zhejiang 310018, P. R. China
First published on 28th July 2015
Abstract
In this paper, the performance of extractive desulfurization (EDS) from gasoline was studied using a mixed solvent, which consisted of N,N-dimethylacetamide (DMAC), N,N-dimethylformamide (DMF) and tetramethylenesulfone (TMS). The effects of relevant parameters on EDS including volume ratio of DMAC/DMF/TMS, extraction temperature, extraction time, stirring speed, volume ratio of extractant and gasoline and initial concentration were investigated. The extraction removal of dibenzothiophene (DBT) and the residual sulfur content reached 99.1% and 9.5 ppm, respectively, at an optimal extractive condition of volume ratio of DMAC/DMF/TMS of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and volume ratio of extractant to gasoline of 1
1 and volume ratio of extractant to gasoline of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 at a stirring speed of 100 rpm over 10 min for extraction at 30 °C (ambient temperature) with five extraction stages. The DMAC/DMF/TMS extractant could be reused for several cycles maintaining high sulfur removal before being regenerated through adsorption. The impacts of three individual process variables such as, extraction time, extraction temperature and volume ratio of extractant to gasoline were investigated using Box–Behnken experimental design and their optimum values were found to be 15 min, 37 °C and 0.5, respectively. These results can be referred to for sulfur removal from gasoline in industrial applications.
5 at a stirring speed of 100 rpm over 10 min for extraction at 30 °C (ambient temperature) with five extraction stages. The DMAC/DMF/TMS extractant could be reused for several cycles maintaining high sulfur removal before being regenerated through adsorption. The impacts of three individual process variables such as, extraction time, extraction temperature and volume ratio of extractant to gasoline were investigated using Box–Behnken experimental design and their optimum values were found to be 15 min, 37 °C and 0.5, respectively. These results can be referred to for sulfur removal from gasoline in industrial applications.
1. Introduction
The deep removal of sulfur-containing compounds from fuel oils has attracted wide interest due to the stringent environmental regulations imposed on sulfur levels in gasoline. The sulfur compounds in petroleum can be converted to sulfur oxide (SOx) and airborne particulate emissions which lead to serious environmental pollution.1 Sulfur could also affect the catalytic converter of vehicles and shorten the life span of the internal combustion engine.2,3 Therefore, the demand for ultra-low-sulfur gasoline is currently huge.4,5Sulfur-containing compounds in petroleum include polysulfides, mercaptans, disulfides, thiophene (TH), dibenzothiophene, benzothiophene (BT), 4,6-dimethyldibenzothiophene (4,6-DMDBT) and their alkylated derivatives. These compounds are very difficult to be removed from fuel. As a traditional desulfurization technology which has been applied conventionally in industry, hydrodesulfurization (HDS) is facing a huge challenge in meeting new stringent regulations and legislations.2,6 This classical process consumes hydrogen, and must be operated at high temperature and pressure.7,8 Therefore, it is highly desired to develop non-HDS methods to produce clean diesel containing extremely low concentration of sulfur under mild conditions and also with low cost. Among ways that has been investigated, EDS has received much attention due to its advantages such as mild operating conditions and no consumption of H2.6,9 EDS is principally based on better solubility of sulfur compounds and aromatic hydrocarbons compared with nonaromatics in appropriate polar solvent.10,11 Also, it does not change the chemical structure of the compounds and consequently has no effect on the quality of liquid fuels.9 Importantly, EDS performs high desulfurization efficiency.12 In the field of oil recovery, Hu et al.13 reported that the solvent extraction as a part of key technology performed good result.
Organic solvents have attracted much attention in the field of extractive desulfurization, because of their low viscosity, unique physical–chemical properties and high regeneration efficiency. Different organic solvents, such as dimethyl sulfoxide (DMSO), acetonitrile and 1-methyl-2-pyrrolidinone (NMP) have been used as extractive solvents in desulfurization.11 Nevertheless, the commonly used solvents are of high toxicity, expensive, ineffective and they have serious consequences for the environment. Therefore, it can be of great significance to explore new cheap, effective and recyclable solvents to improve the extractive desulfurization process.
N,N-Dimethylacetamide (DMAC) is a colorless and transparent nonproton solvent with high polarity.14 It has an applicability over a wide temperature range owing to its high boiling point (>160 °C). It has been widely used in industries because of its numerous excellent properties, such as good solubility, high hydrothermal stability, difficult hydrolysis, and so on.14 Another extractant of N,N-dimethylformamide (DMF), is an aprotic solvent and the boiling point is 153 °C. Pioneer works reported DMF solvent as an excellent polar solvent for various classes of compounds, the dissolution being favored by interactions of the substrate with DMF.15–17 Mokhtar et al.6 found that the utilization of DMF for the desulfurization of DBT achieved a good result. Tetramethylene sulfone (TMS) is a polar solvent with rather good selectivity and the high boiling point of 285 °C makes it better thermal stability.
Generally, the multi-extraction system of EDS performs a better desulfurization at a shorter extraction time, lower stirring speed, and especially higher sulfur extraction efficiency than single extraction. An explanation for this is the cooperative formation mechanism among extractants. Hassan et al.18 claimed that DMF exhibited more efficient extraction solvent characteristics in the addition of ethylene glycol.
In this study, the extractive desulfurization was developed, which was conducted by DMAC, DMF and TMS. Their mixture was treated to be the primary extractant. Our study provided an example of the mixed solvent, of which we particularly focus on the positive effect in the extractive desulfurization. Effects of some important parameters on desulfurization were investigated. A Box–Behnken design was applied to determine the optimum S-extraction efficiency and yield rate, and also to explain the relations between sulfur removal and three pertinent parameters, namely, extraction time, temperature and solvent/model gasoline volume ratio.
2. Experimental
2.1. Materials
All the chemicals were of analytical grade and used as received. DBT (>98%) was the product of Beijing Bailingwei Technology Co. Ltd. (China); BT (>98%) was the product of Beijing Bailingwei Technology Co. Ltd (China); n-octane was purchased from Tianjin Kemiou Chemical Reagent Co. Ltd. (China); DMAC was purchased from Tianjin Fuyu Fine Chemistry Co. Ltd. (China); DMF was purchased from Sinopharm Group Chemical Reagent Co. Ltd. (China); TMS was obtained from Shanghai Crystal Pure Reagent Co. Ltd. (China).2.2. Procedures
The model gasoline was prepared by dissolving certain amount of DBT in n-octane to obtain a solution with initial sulfur concentration of 1000 ppm, and then the solution was submitted to the extractive conditions, as described below. The mixed extractant was prepared by DMAC, DMF and TMS with the volume rate of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
The extractive experiments were carried out at atmospheric pressure, at a constant temperature (between 30 and 60 °C), in an Erlenmeyer flask (100 mL). Then the reactor was placed in a stirred thermostatted shaker. The typical extraction procedure was as follows: 10 mL of model gasoline and the calculated volume of mixed extractant were mixed together in different volume ratios of mixed extractant and gasoline (0.5–2.5). Following this, the binary mixture was stirred at 100 rpm, stopped at desired time intervals and sampling was conducted for further quantification. The extraction time ranged from 2 min to 20 min, then held for 15 min.
To achieve ultra-deep desulfurization, same process could be repeated several times with the total amount of extractant remaining unchanged. This experimental procedure consisted of extraction and separation, which was modified from the procedure established by Mokhtar et al.6 The used extractant was reused several cycles for fresh gasoline and then regenerated by a simple adsorption method. All experiments were repeated three times to secure reproducibility of results.
2.3. Analysis
DBT and BT concentration in samples were analyzed using gas chromatography (GC) (Agilent 6890 N, USA) equipped with a flame ionization detector (FID, HP6890). A HP-5 capillary column (30 m × 0.32 mm × 0.25 μm film thickness) was used for separation. Highly purified nitrogen (mass concentration ≥ 99.9999%) was used as carrier gas.The sulfur removal was calculated to evaluate the activity of the ternary extraction system. Reaction rates equations for extractive desulfurization was calculated using eqn (1), where η is the extraction rate, and C0 and Ct represent the initial and final sulfur content in model gasoline, respectively.
| η = [(C0 − Ct)/C0] × 100% | (1) |
Yield rate of model gasoline equation is shown in eqn (2), in which λ is the yield rate, and m0 and mt stand for the initial and final weight of model gasoline, respectively.
| λ = [(m0 − mt)/m0] × 100% | (2) |
2.4. Experimental design
The DBT removal and gasoline yield rate were optimized by response surface methodology using Box–Behnken Design (BBD). The statistical software Design Expert 8.0.5 was used for the analysis. Three independent parameters, extraction time, X1 (2–20 min), extraction temperature, X2 (30–60 °C) and solvent/model gasoline volume ratio, X3 (0.5–2.5) were confirmed to optimize the DBT removal and yield rate. The coded and uncoded levels of these variables were presented in Table 1. With statistical analysis of the gained experimental data, a quadratic equation (eqn (3)) was attained as an empirical model for the optimization process.| Y = β0 + ∑βiXi + ∑βiiXi2 + ∑βijXiXj | (3) |
| Independent variables | Range and levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Extraction time, X1 (min) | 2 | 11 | 20 |
| Extraction temperature, X2 (°C) | 30 | 45 | 60 |
| Solvent/model gasoline volume ratio, X3 | 0.5 | 1.5 | 2.5 |
3. Results and discussion
3.1. Effects of different extractants on DBT removal
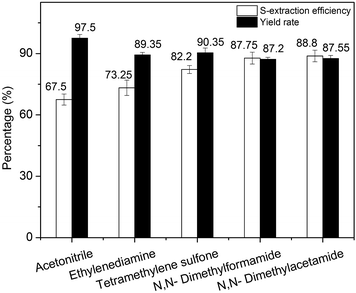
Effects of five different kinds of traditional organic polar solvents applying to extractive desulfurization on DBT removal were evaluated at the following conditions: extraction temperature of 30 °C, extraction and holding time were 10 and 15 min, respectively. The stirring speed of 100 rpm, volume of the model gasoline was 10 mL and volume ratio of extractant and gasoline was 1.0. In addition, the extraction process of sulfur content was conducted with one stage. As shown in Fig. 1, DMAC exhibited higher extractive activity than the other four. However, for EDS, TMS as solvent made the yield rate of the model gasoline exceeding 90.0% and the desulfurization efficiency exceeded 80%. Hereinafter, DMAC, DMF, TMS were chosen as the representative solvents for next investigation.Next, three kinds of solvents and their mixture (volume proportion of DMAC/DMF/TMS = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) had been used to desulfurization, respectively. As can be seen in Fig. 2a, a favourable effect of the mixed extractant was obtained with the highest sulfur extraction efficiency of 92.5 ± 3.0% in 10 min. Accordingly, the efficiencies were 88.8 ± 2.8%, 87.8 ± 2.9%, 82.2 ± 2.0% for DMAC, DMF and TMS, respectively. Probably, once they were present as a mixture, synergic effects could be operative, to facilitate the extraction. That is, these results were possibly due to the occurrence of solvent synergism.19,20 Therefore, the mixed solvent was selected for the successive experiments.
1) had been used to desulfurization, respectively. As can be seen in Fig. 2a, a favourable effect of the mixed extractant was obtained with the highest sulfur extraction efficiency of 92.5 ± 3.0% in 10 min. Accordingly, the efficiencies were 88.8 ± 2.8%, 87.8 ± 2.9%, 82.2 ± 2.0% for DMAC, DMF and TMS, respectively. Probably, once they were present as a mixture, synergic effects could be operative, to facilitate the extraction. That is, these results were possibly due to the occurrence of solvent synergism.19,20 Therefore, the mixed solvent was selected for the successive experiments.
To evaluate the effect of the volume ratio of DMAC, DMF and TMS on extraction process, the EDS experiments were conducted under different ratios and the results were shown in Fig. 2b. The results indicated that the proportion of DMAC played a most important role on extraction. When the volume ratio of DMAC to total volume was varied from 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 0.6
1 to 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the extraction efficiency was increased and reached up to 93.0 ± 0.8% at 10 min. Meanwhile, the extraction efficiency was increased and reached up to 90.8 ± 0.8% and 86.8 ± 0.4% at 10 min for DMF and TMS, respectively. Hence, the volume ratio of DMAC, DMF and TMS was set at 3
1, the extraction efficiency was increased and reached up to 93.0 ± 0.8% at 10 min. Meanwhile, the extraction efficiency was increased and reached up to 90.8 ± 0.8% and 86.8 ± 0.4% at 10 min for DMF and TMS, respectively. Hence, the volume ratio of DMAC, DMF and TMS was set at 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the consequent experiments.
1 in the consequent experiments.
3.2. Effects of extraction conditions on DBT removal
The yield rate of the model gasoline decreased slightly when the reaction temperature increased from 30 to 45 °C (Fig. 3a). However, it decreased sharply from 89.7 ± 3.8% to 83.0 ± 1.4% when the temperature was further increased to 60 °C. In the meanwhile, it can be seen from Fig. 3b that the yield rate of model gasoline with no sulfur decreased from nearly 100% to 94.5 ± 0.7% when the temperature was increased from 30 to 60 °C. Consequently, evaporative losses of model gasoline were obvious at higher temperatures. Taken together, the temperature of 30 °C was suitable for this extraction system of DMAC/DMF/TMS, because this extraction system showed excellent desulfurization efficiency at this point and it was close to room temperature. The similar results had been reported by other published works.22–24 However, there were different results using some ILs, in which, the extraction efficiencies increased and then decreased with the increase of the temperature.25 The temperature dependency of extraction with ILs may be attributed to their high viscosity.25
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Further increase of the solvent/model gasoline volume ratio to 2.5
1. Further increase of the solvent/model gasoline volume ratio to 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 led to 96.6 ± 3.2% of DBT removed. Nevertheless, the yield rate of model gasoline decreased dramatically from 90.2 ± 2.3% to 36.2 ± 2.5% with the increase of solvent/model gasoline volume ratio from 1
1 led to 96.6 ± 3.2% of DBT removed. Nevertheless, the yield rate of model gasoline decreased dramatically from 90.2 ± 2.3% to 36.2 ± 2.5% with the increase of solvent/model gasoline volume ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2.5
1 to 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. These were possibly due to the occurrence of compatibility of the model gasoline and extractant. Furthermore, excess volume of solvent deserved a higher cost of the extraction and recovery process as well.6 Thus, 1
1. These were possibly due to the occurrence of compatibility of the model gasoline and extractant. Furthermore, excess volume of solvent deserved a higher cost of the extraction and recovery process as well.6 Thus, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was chosen as the operation volume ratio throughout the investigation. Kianpour et al.9 reported that volume ratio of polyethylene glycol to model fuel of 1
1 was chosen as the operation volume ratio throughout the investigation. Kianpour et al.9 reported that volume ratio of polyethylene glycol to model fuel of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was selected for the EDS, and Mokhtar et al.6 found that the DMF/model diesel ratio of 1
1 was selected for the EDS, and Mokhtar et al.6 found that the DMF/model diesel ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was the best ratio.
1 was the best ratio.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
3.3. Optimization by Box–Behnken design
The Box–Behnken design was applied in the study and 17-experimental runs were conducted at orders randomly for the optimization of DBT removal and gasoline yield rate in the extractive desulfurization procedure. Table 2 presented the data resulting from the experiments including the coded values of the parameters (−1, 0 and +1), their actual values, and the corresponding responses (predicted values). Three variables of extraction time (X1), extraction temperature (X2) and solvent/model gasoline volume ratio (X3) and the experimental results were analyzed by means of RSM to get an empirical model for the best response. In this procedure, stirring speed and extraction stage remained unchanged at 100 rpm and 1, respectively. The final second-order polynomial was attained to explain the mathematical relation between the independent parameters and the dependent responses (Y). They were presented below:| S-extraction efficiency (Y1) = 94.92 + 11.44X1 − 1.37X2 + 4.86X3 + 0.25X1X2 − 0.075X1X3 + 0.00X2X3 − 10.52X12 − 1.17X22 − 3.35X32 |
| Yield rate (Y2) = 62.59 − 2.90X1 − 3.70X2 − 30.78X3 + 0.00X1X2 − 0.40X1X3 + 0.00X2X3 − 0.17X12 − 3.62X22 + 4.26X32 |
| Run | Coded values | Actual values | S-extraction efficiency | Yield rate | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X1 | X2 | X3 | Yexp | Ypred | Residual | Yexp | Ypred | Residual | |
| 1 | −1 | 0 | 1 | 2 | 45 | 2.5 | 74.35 | 74.55 | −0.20 | 38.95 | 39.20 | −0.25 |
| 2 | 0 | −1 | −1 | 11 | 30 | 0.5 | 86.75 | 86.91 | −0.16 | 97.65 | 97.70 | −0.050 |
| 3 | −1 | 1 | 0 | 2 | 60 | 1.5 | 70.20 | 70.16 | 0.038 | 58.2 | 58.00 | 0.20 |
| 4 | 0 | −1 | 1 | 11 | 30 | 2.5 | 96.55 | 96.64 | −0.087 | 36.2 | 36.15 | 0.050 |
| 5 | 1 | 0 | 1 | 20 | 45 | 2.5 | 97.40 | 97.28 | 0.13 | 32.75 | 32.60 | 0.15 |
| 6 | 0 | 1 | −1 | 11 | 60 | 0.5 | 84.25 | 84.16 | 0.087 | 90.25 | 90.30 | −0.050 |
| 7 | 1 | −1 | 0 | 20 | 30 | 1.5 | 95.75 | 95.79 | −0.037 | 59.4 | 59.60 | −0.20 |
| 8 | 0 | 0 | 0 | 11 | 45 | 1.5 | 94.90 | 94.92 | −0.020 | 62.45 | 62.59 | −0.14 |
| 9 | 0 | 0 | 0 | 11 | 45 | 1.5 | 94.80 | 94.92 | −0.12 | 62.55 | 62.59 | −0.036 |
| 10 | 0 | 1 | 1 | 11 | 60 | 2.5 | 94.05 | 93.89 | 0.16 | 28.8 | 28.75 | 0.050 |
| 11 | 1 | 1 | 0 | 20 | 60 | 1.5 | 93.25 | 93.54 | −0.29 | 52 | 52.20 | −0.20 |
| 12 | −1 | −1 | 0 | 2 | 30 | 1.5 | 73.70 | 73.41 | 0.29 | 65.6 | 65.40 | 0.20 |
| 13 | 1 | 0 | −1 | 20 | 45 | 0.5 | 87.90 | 87.70 | 0.20 | 95.2 | 94.95 | 0.25 |
| 14 | −1 | 0 | −1 | 2 | 45 | 0.5 | 64.55 | 64.67 | −0.12 | 99.8 | 99.95 | −0.15 |
| 15 | 0 | 0 | 0 | 11 | 45 | 1.5 | 95.10 | 94.92 | 0.18 | 62.5 | 62.59 | −0.086 |
| 16 | 0 | 0 | 0 | 11 | 45 | 1.5 | 94.90 | 94.92 | −0.020 | 62.68 | 62.59 | 0.094 |
| 17 | 0 | 0 | 0 | 11 | 45 | 1.5 | 94.90 | 94.92 | −0.020 | 62.75 | 62.59 | 0.16 |
Additionally, the results related with variance (ANOVA) analysis, shown in Tables 3and 4 illustrated the successful fitting of the experiment data to the quadratic model. The model F-values of 3534.98 for S-extraction efficiency and 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 209.75 for yield rate implied the model was significant. There was only a 0.01% chance that a “Model F-Value” this large could occur due to noise. This indicated that the assumed second order polynomial (eqn (3)) was highly significant. Value of P less than 0.0500 indicated model terms were significant. In this case X1, X2, X3, X12, X22 and X32 in Table 3 and X1, X2, X3, X1X3, X22 and X32 in Table 4 were significant model terms. From Table 3, extraction time was the most influential parameter for S-extraction efficiency, which achieved 18522.68 of F-value. However, solvent/model gasoline volume ratio had a maximum impact on yield rate with F-value of 131
209.75 for yield rate implied the model was significant. There was only a 0.01% chance that a “Model F-Value” this large could occur due to noise. This indicated that the assumed second order polynomial (eqn (3)) was highly significant. Value of P less than 0.0500 indicated model terms were significant. In this case X1, X2, X3, X12, X22 and X32 in Table 3 and X1, X2, X3, X1X3, X22 and X32 in Table 4 were significant model terms. From Table 3, extraction time was the most influential parameter for S-extraction efficiency, which achieved 18522.68 of F-value. However, solvent/model gasoline volume ratio had a maximum impact on yield rate with F-value of 131![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 (Table 4). The quite high R2 (R-Sq) values of 0.9998 for S-extraction efficiency and 0.9999 for yield rate indicated that the predicted polynomial model was reasonably well fitted with the data. The predicted R2 (Pred. R-Sq) values of 0.9969 for S-extraction efficiency and 0.9993 for yield rate were in reasonable agreement with the adjusted R2 (Adj. R-Sq) values of 0.9995 for S-extraction efficiency and 0.9999 for yield rate. The comparisons between experimental and predicted values of S-extraction efficiency and yield rate (%) were exhibited graphically with 45° C-lines respectively in Fig. 10. Very little deviations were discovered between points that represented experimental values and the regression line that represented predicted values.
600 (Table 4). The quite high R2 (R-Sq) values of 0.9998 for S-extraction efficiency and 0.9999 for yield rate indicated that the predicted polynomial model was reasonably well fitted with the data. The predicted R2 (Pred. R-Sq) values of 0.9969 for S-extraction efficiency and 0.9993 for yield rate were in reasonable agreement with the adjusted R2 (Adj. R-Sq) values of 0.9995 for S-extraction efficiency and 0.9999 for yield rate. The comparisons between experimental and predicted values of S-extraction efficiency and yield rate (%) were exhibited graphically with 45° C-lines respectively in Fig. 10. Very little deviations were discovered between points that represented experimental values and the regression line that represented predicted values.
| Source | SSb | DFb | MSb | Fb | Pb | CEb |
|---|---|---|---|---|---|---|
| a R-Sq = 99.98%; R-Sq (Adj.) = 99.95%; R-Sq (Pred.) = 99.69%.b SS: sum of square; DF: degree of freedom of different source; MS: mean of square; F: degree of freedom; P: probability; CE: coefficient estimate. | ||||||
| Modela | 1797.54 | 9 | 199.73 | 3534.98 | <0.0001 | |
| Time, X1 | 1046.53 | 1 | 1046.53 | 18522.68 | <0.0001 | 11.44 |
| Temperature, X2 | 15.13 | 1 | 15.13 | 267.70 | <0.0001 | −1.37 |
| Volume ratio, X3 | 189.15 | 1 | 189.15 | 3347.81 | <0.0001 | 4.86 |
| X1X2 | 0.25 | 1 | 0.25 | 4.42 | 0.0735 | 0.25 |
| X1X3 | 0.023 | 1 | 0.023 | 0.40 | 0.5480 | −0.075 |
| X2X3 | 0.000 | 1 | 0.000 | 0.000 | 1.0000 | 0.000 |
| X12 | 466.20 | 1 | 466.20 | 8251.37 | <0.0001 | −10.52 |
| X22 | 5.79 | 1 | 5.79 | 102.45 | <0.0001 | −1.17 |
| X32 | 47.18 | 1 | 47.18 | 835.08 | <0.0001 | −3.35 |
| Residual | 0.40 | 7 | 0.056 | |||
| Lack of fit | 0.35 | 3 | 0.12 | 9.65 | 0.0265 | |
| Pure error | 0.048 | 4 | 0.012 | |||
| Cor. total | 1797.93 | 16 | ||||
| Source | SSb | DFb | MSb | Fb | Pb | CEb |
|---|---|---|---|---|---|---|
| a R-Sq = 99.99%; R-Sq (Adj.) = 99.99%; R-Sq (Pred.) = 99.93%.b SS: sum of square; DF: degree of freedom of different source; MS: mean of square; F: degree of freedom; P: probability; CE: coefficient estimate. | ||||||
| Modela | 7879.26 | 9 | 875.47 | 15209.75 | <0.0001 | |
| Time, X1 | 67.28 | 1 | 67.28 | 1168.87 | <0.0001 | −2.90 |
| Temperature, X2 | 109.52 | 1 | 109.52 | 1902.71 | <0.0001 | −3.70 |
| Volume ratio, X3 | 7576.81 | 1 | 7576.81 | 1.316 ×105 | <0.0001 | −30.78 |
| X1X2 | 0.000 | 1 | 0.000 | 0.000 | 1.0000 | 0.000 |
| X1X3 | 0.64 | 1 | 0.64 | 11.12 | 0.0125 | −0.40 |
| X2X3 | 0.000 | 1 | 0.000 | 0.000 | 1.0000 | 0.000 |
| X12 | 0.12 | 1 | 0.12 | 2.06 | 0.1939 | −0.17 |
| X22 | 55.12 | 1 | 55.12 | 957.53 | <0.0001 | −3.62 |
| X32 | 76.30 | 1 | 76.30 | 1325.63 | <0.0001 | 4.26 |
| Residual | 0.40 | 7 | 0.058 | |||
| Lack of fit | 0.34 | 3 | 0.11 | 7.20 | 0.0433 | |
| Pure error | 0.063 | 4 | 0.016 | |||
| Cor. total | 7879.66 | 16 | ||||
 | ||
| Fig. 10 Comparison of the experimental results of S-extraction efficiency (a) and gasoline yield rate (b) with those calculated via Box–Behnken design (BBD) resulted equation. | ||
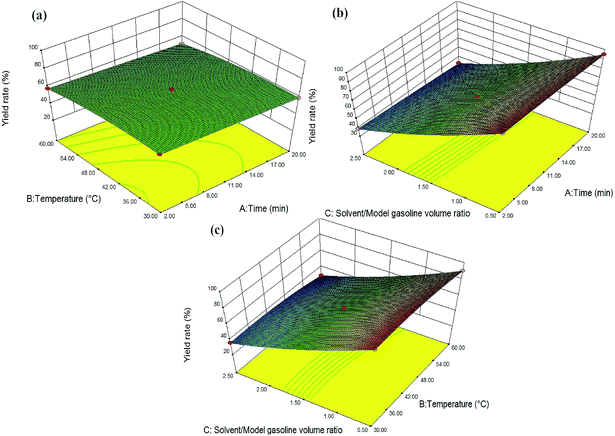
The significance of each of three independent factors (extraction time, temperature and solvent/model gasoline volume ratio) on S-extraction efficiency and yield rate was determined by illustrating the response surfaces as three dimensional (3D) plots (Fig. 11 and 12). The solvent/model gasoline volume ratio was kept a constant at 1.5 (Fig. 11a and 12a), while the extraction temperature and time were kept constants at 45 °C (Fig. 11b and 12b) and 11 min (Fig. 11c and 12c), respectively. As shown in Fig. 11a, S-extraction efficiency increased with the increasing of extraction time at lower extraction temperature. The highest sulfur removal (>90%) occurred when extraction time and temperature were stayed at about 14–16 min and 36–40 °C, respectively. Meanwhile, in Fig. 11b, the variations of extraction time dramatically affected the DBT removal, while the variations of solvent/model gasoline volume ratio were less important. Fig. 11c illustrated the effect of extraction temperature and solvent/model gasoline volume ratio on sulfur removal. Obviously, the variation of solvent/model gasoline volume ratio was more important than extraction temperature. Above all, the degree of importance of the three parameters on DBT removal was: extraction time > solvent/model gasoline volume ratio > extraction temperature.
As can be seen from Fig. 12a, the extraction temperature and extraction time had a slight effect on the yield rate. In Fig. 12b, the effect of the solvent/model gasoline volume ratio on yield rate was more significant compared with the extraction time. Fig. 12c demonstrated the influence of extraction temperature and solvent/model gasoline volume ratio on yield rate at the extraction time of 11 min. It was obvious that the variation of solvent/model gasoline volume ratio was more important than extraction temperature. Overall, the degree of importance of the three parameters on yield rate was: solvent/model gasoline volume ratio > extraction temperature > extraction time.
Response optimization technique helped to identify a production of a combination of input variables that collectively optimized a single response or a set of responses. The particular desirability of both the variance and the seal strength was 1.0, which indicated that the combined desirability of these two variables was also 1.0.30 In order to obtain the desirability, the factor levels were set at the values given to maximize the S-extraction efficiency and yield rate by adjusting at the starting point of optimization. The values of the process variables for the maximum rate were presented in Table 5. The optimum values of the independent variables were attained by considering the starting values of extraction time, temperature and solvent/model gasoline volume ratio of 10 min, 30 °C and 1.0, respectively. The maximum S-extraction efficiency and yield rate of 90.2% and 97.3% respectively could be estimated by choosing the optimum extraction time of 15 min, extraction temperature of 37 °C with solvent/model gasoline volume ratio of 0.5. Therefore, the RSM could be successfully applied to maximize the DBT removal and yield rate of gasoline. In order to confirm the agreement of the model and experimental results, an additional experiment was carried out under the optimum conditions. The experimental values (91.0% for S-extraction efficiency and 95.1% for yield rate) were in great agreement with the predicted result and thus validated the findings of response surface optimization.
| Parameter | Values |
|---|---|
| a Composite desirability = 1.000000. | |
| S-extraction efficiency, % | 90.2 |
| Yield rate, % | 97.3 |
| X1 (extraction time, min) | 15 |
| X2 (extraction temperature, °C) | 37 |
| X3 (solvent/model gasoline volume ratio) | 0.5 |
3.4. Reuse of DMAC/DMF/TMS and regeneration of spent DMAC/DMF/TMS
In order to obtain the information on the stability of DMAC/DMF/TMS system, reuse of the spent solvent for extraction desulfurization was investigated. As shown in Fig. 13, extraction capability of DMAC/DMF/TMS system decreased as the increase of repeated use. After three cycles, the desulfurization rates were less than 70% for DBT-octane solution. This indicated that the higher extraction capability of the DMAC/DMF/TMS extraction system was lost and it must be regenerated. However, after three cycles, the extraction efficiency of DBT was about 10 percentage higher than the research which used polyethylene glycol as extractant for desulfurization.9The effect of regenerated extractant was important for industrial applications. In general, the extracted polar organic solvent could be recovered by using any conventional separation method, for instance, distillation, adsorption and back-extraction processes.31 Note that distillation was the main recycling method for ILs, but the cost of this technique was higher.26 In this context, the spent extractant was regenerated by adsorption method, which was similar to the study of DBT removal by polyethylene glycol.9 From Fig. 9, the extraction ability of the extractant regenerated by powder 4A molecular sieve (volume mass ratio of spent extractant/adsorbent 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) increased from 65.0 ± 1.4% to 85.3 ± 1.5% for DBT removal. The results indicated that the regenerated extractant had a very good recycling performance in the desulfurization.
1) increased from 65.0 ± 1.4% to 85.3 ± 1.5% for DBT removal. The results indicated that the regenerated extractant had a very good recycling performance in the desulfurization.
4. Conclusions
The DMAC/DMF/TMS system was highly effective for extraction of dibenzothiophene from gasoline. This system reached high extraction efficiency of 92.5% for DBT at the optimal extractive condition of a volume ratio of DMAC/DMF/TMS of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a volume ratio of the extractant to model gasoline of 1
1, a volume ratio of the extractant to model gasoline of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at a stirring speed of 100 rpm over 10 min for extraction at 30 °C (ambient temperature) with one extraction stage. The sulfur content reduced from 1000 to 9.5 ppm (99.1%) within five extraction stages, 1
1 at a stirring speed of 100 rpm over 10 min for extraction at 30 °C (ambient temperature) with one extraction stage. The sulfur content reduced from 1000 to 9.5 ppm (99.1%) within five extraction stages, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
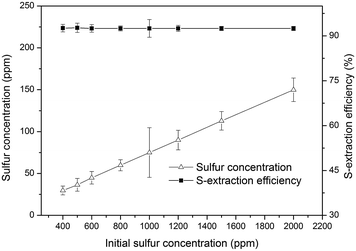
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 of extractant to gasoline by volume for each stage. Moreover, Sulfur extraction efficiency of DMAC/DMF/TMS was nearly independent of initial sulfur content at the above optimal conditions.
5 of extractant to gasoline by volume for each stage. Moreover, Sulfur extraction efficiency of DMAC/DMF/TMS was nearly independent of initial sulfur content at the above optimal conditions.
The results were verified by Box–Behnken experimental design. Among the three relevant variables (extraction time, extraction temperature and solvent/model gasoline volume ratio), extraction time and solvent/model gasoline volume ratio were the most influential parameters for S-extraction efficiency and yield rate, respectively. The model equation attained using BBD presented the high coefficient of determination (R12 = 0.9998 and R22 = 0.9999) indicating that the predicted data fitted well with the experimental data. On the basis of the statistical design method, the optimal operation conditions were determined at extraction time = 15 min, extraction temperature = 37 °C and solvent/model gasoline volume ratio = 0.5. The experiment for verification was conducted under the optimum conditions and the actual values (91.0% for S-extraction efficiency and 95.1% for yield rate) nearly agreed with predicted values (90.2% for S-extraction efficiency and 97.3% for yield rate).
Then DMAC/DMF/TMS was reused three cycles and spent DMAC/DMF/TMS was regenerated by adsorption method. Regenerated extractant could effectively extract DBT from fresh model gasoline with extraction efficiency of 85.3%.
The DMAC/DMF/TMS extraction system shows the potential to overcome the disadvantages of existing technologies, and could be a cost-effective process for ultra-deep desulfurization.
Acknowledgements
Financial support from the Nation Science Foundation of China (Grant number: 51278464 and 51478172), the International S&T Cooperation Program of China (Project Contract no. 2015DFG92750), and Department of Science & Technology of Hunan Province (Project Contract no. 2014GK1012) is greatly appreciated.References
- J. M. Campos-Martin, M. C. Capel-Sanchez, P. Perez-Presas and J. L. G. Fierro, J. Chem. Technol. Biotechnol., 2010, 85, 879–890 CrossRef CAS PubMed.
- Z. Y. Long, C. P. Yang, G. M. Zeng, L. Y. Peng, C. H. Dai and H. J. He, Fuel, 2014, 130, 19–24 CrossRef CAS PubMed.
- B. Pawelec, R. M. Navarro, J. M. Campos-Martin and J. L. G. Fierro, Catal. Sci. Technol., 2013, 3, 3376 CAS.
- I. V. Babich and J. A. Moulijn, Fuel, 2003, 82, 607–631 CrossRef CAS.
- C. Song and X. L. Ma, Appl. Catal., B, 2003, 41, 207–238 CrossRef CAS.
- W. Mokhtar, W. A. W. Abu Bakar, R. Ali and A. A. A. Kadir, J. Taiwan Inst. Chem. Eng., 2014, 45, 1542–1548 CrossRef CAS PubMed.
- C. Y. Jiang, J. J. Wang, S. T. Wang, H. Y. Guan, X. H. Wang and M. X. Huo, Appl. Catal., B, 2011, 106, 343–349 CrossRef CAS PubMed.
- N. D. McNamara, G. T. Neumann, E. T. Masko, J. A. Urban and J. C. Hicks, J. Catal., 2013, 305, 217–226 CrossRef CAS PubMed.
- E. Kianpour and S. Azizian, Fuel, 2014, 137, 36–40 CrossRef CAS PubMed.
- T. Adzamic, K. Sertic-Bionda and N. Marcec-Rahelic, Pet. Sci. Technol., 2010, 28, 1936–1945 CrossRef CAS PubMed.
- L. L. Ban, P. Liu, C. H. Ma and B. Dai, Chin. Chem. Lett., 2013, 24, 755–758 CrossRef CAS PubMed.
- C. P. Li, D. Li, S. S. Zou, Z. Li, J. M. Yin, A. L. Wang, Y. N. Cui, Z. L. Yao and Q. Zhao, Green Chem., 2013, 15, 2793–2799 RSC.
- J. Li, G. Hu and H. Hou, J. Hazard. Mater., 2015, 283, 832–840 CrossRef PubMed.
- C. X. Wang, X. J. Liu, P. Wang and P. F. Yang, J. Chem. Eng. Data, 2014, 59, 1733–1736 CrossRef CAS.
- J. Muzart, Tetrahedron, 2009, 65, 8313–8323 CrossRef CAS PubMed.
- S. Otsuki, T. Nonaka, N. Takashima, W. H. Qian, A. Ishihara, T. Imai and T. Kabe, Energy Fuels, 2000, 14, 1232–1239 CrossRef CAS.
- L. Topalova, V. Toteva and P. Manolova, J. Univ. Chem. Technol. Metall., 2007, 42, 17–20 Search PubMed.
- O. I. Sif EI-Din, S. I. Hassan and S. M. Tawfik, J. Appl. Sci. Res., 2009, 5, 515–521 Search PubMed.
- D. W. Behnken and G. E. P. Box, Technometrics, 1960, 2, 455–475 CrossRef PubMed.
- J. V. Oliveira, L. Conceicao, W. F. Souza and S. B. C. Pergher, Brazilian Journal of Petroleum and Gas, 2009, 3, 159–165 Search PubMed.
- F. T. Li, C. G. Kou, Z. M. Sun, Y. J. Hao, R. H. Liu and D. S. Zhao, J. Hazard. Mater., 2012, 205, 164–170 CrossRef PubMed.
- C. Asumana, G. R. Yu, X. Li, J. J. Zhao, G. Liu and X. C. Chen, Green Chem., 2010, 12, 2030–2037 RSC.
- X. C. Chen, S. Yuan, A. A. Abdeltawab, S. S. Al-Deyab, J. W. Zhang, L. Yu and G. R. Yu, Sep. Purif. Technol., 2014, 133, 187–193 CrossRef CAS PubMed.
- C. D. Wilfred, C. F. Kiat, Z. Man, M. A. Bustam, M. I. M. Mutalib and C. Z. Phak, Fuel Process. Technol., 2012, 93, 85–89 CrossRef CAS PubMed.
- S. A. Dharaskar, K. L. Wasewar, M. N. Varma, D. Z. Shende, K. K. Tadi and C. K. Yoo, Fuel Process. Technol., 2014, 123, 1–10 CrossRef CAS PubMed.
- Z. Li, C. P. Li, Y. S. Chi, A. L. Wang, Z. D. Zhang, H. X. Li, Q. S. Liu and U. Welz-Biermann, Energy Fuels, 2012, 26, 3723–3727 CrossRef CAS.
- R. N. Fallah and S. Azizian, Fuel Process. Technol., 2012, 93, 45–52 CrossRef CAS PubMed.
- W. Jiang, W. S. Zhu, Y. H. Chang, Y. H. Chao, S. Yin, H. Liu, F. X. Zhu and H. M. Li, Chem. Eng. J., 2014, 250, 48–54 CrossRef CAS PubMed.
- K. A. Cychosz, A. G. Wong-Foy and A. J. Matzger, J. Am. Chem. Soc., 2008, 130, 6938–6939 CrossRef CAS PubMed.
- N. Jose, S. Sengupta and J. K. Basu, Fuel, 2011, 90, 626–632 CrossRef CAS PubMed.
- A. R. Ferreira, M. G. Freire, J. C. Ribeiro, F. M. Lopes, J. G. Crespo and J. A. P. Coutinho, Fuel, 2014, 128, 314–329 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |