DNA protection, antioxidant, antibacterial and enzyme inhibition activities of heartwood and sapwood extracts from juniper and olive woods
Osman Emre Özkana,
Gökhan Zenginb,
Mehmet Akçaa,
Mehmet Cengiz Baloğlu*c,
Çağrı Olguna,
Ergin Murat Altunerd,
Saim Ateşa,
Abdurrahman Aktümsekb and
Hasan Vurdua
aDepartment of Forest Industrial Engineering, Faculty of Forestry, Kastamonu University, Kastamonu, 37150, Turkey
bDepartment of Biology, Science Faculty, Selcuk University, Konya, Turkey
cDepartment of Genetics and Bioengineering, Faculty of Engineering and Architecture, Kastamonu University, Kastamonu, 37150, Turkey. E-mail: mcbaloglu@gmail.com
dDepartment of Biology, Faculty of Science, Kastamonu University, Kastamonu, 37150, Turkey
First published on 21st August 2015
Abstract
In this study, DNA protective, antioxidant, antibacterial and enzyme inhibiting properties of methanol extracts obtained from juniper and olive heartwood and sapwood were determined. These extracts were tested by five antioxidant methods (DPPH scavenging, FRAP, CUPRAC, metal chelating and phosphomolybdenum). Generally, heartwood extracts of both species are more efficient for DPPH radical scavenging activity, cupric ion reducing activity, ferric reducing antioxidant power and metal chelating activity than sapwood extracts. When compared to heartwood extracts, sapwood extracts have larger inhibition zone in disk diffusion test. In addition, all extracts showed high antibacterial activity against Staphylococcus aureus. DNA protection of both extracts had a capacity to inhibit the DNA damage arisen from Fenton's reagent. The highest DNA protective activity was observed in juniper sapwood extract with 84%. Furthermore, other extracts also indicated more than 60% of DNA protective activity. Olive wood extracts displayed the strongest enzyme inhibition activities against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). Although juniper heartwood extracts showed highest anti-amylase, anti-glucosidase and anti-tyrosinase effects, they had no ability for inhibition BChE. The methanol extracts of olive samples demonstrated the most antioxidant activity (DPPH, CUPRAC and FRAP). In addition, juniper samples showed the highest anti-amylase, anti-tyrosinase, metal chelating and DNA protective activity. According to these results, the extracts of juniper and olive wood can be considered as a source of natural bio active agents for dietary, pharmacological and medicinal applications. This research will also serve as a base for future studies about biological activities of wood extracts.
1. Introduction
In recent years, people have been interested in obtaining active compounds from natural sources. Wood extracts have served as an important source of bioactive compounds. Specially, naturally durable wood species juniper and olive, could be a rich source of bioactive compounds. Juniperus foetidissima Willd. belongs to Juniperus genus which is composed of 67 species and 34 varieties. They are evergreen bushes or trees and mostly widespread in the Northern Hemisphere.1 In Turkey, 10 taxa of Juniperus have been distributed from Mediterranean coastal to Taurus Mountains and also central and northern parts of Anatolia.2 Juniperus species are well-known in the traditional medicine of ancient civilizations of Europe and Asia due to their numerous pharmacological properties. Juniper berries and leaves are mainly used for diuretic, antiseptic, carminative, stomachic, antirheumatic, antifungal and disinfectant properties in folk medicines.3 Karaman et al.4 reported that methanol extracts of J. oxycedrus L. have antibacterial and anticandical properties. Juniperus species are also used for cosmetic5 and food industries.6 Oil of cade, known as “juniper tar” produced from J. oxycedrus, has been widely utilized for dermatology to heal eczema and other skin diseases. In addition, this oil has been added into some cosmetic products such as detergents, soaps, creams and lotions.7The olive tree (Olea europaea var. sylvestris Mill.) is one of the most valuable trees in the Mediterranean region. Waste wood from cutting and pruning of olive tree has importance as a bio product. The chemical composition and antioxidant activity of olive wood have been studied in which oleuropein was identified from olive leaves and fruits.8 The antioxidant, antimicrobial, antiviral, and antitumor activities of oleuropein has been reported in different studies.9–12 In addition to leaf and fruit extracts, virgin olive oil has also found a protective effect against nuclear DNA damage in HeLa cells.13
Drug resistance in microorganisms has developed due to misuse of antimicrobial drugs. This situation motivated scientists for searching new antimicrobial substances from various natural sources, like medicinal plants, which are the good sources of novel antimicrobial agents.4 Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are produced in living cells by different cases.14 Exogenous sources of free radicals include tobacco smoke, ionizing radiation, certain pollutants, organic solvents, and pesticides.15 ROS and RNS may cause DNA damage that could lead to mutation. All aerobic organisms, including human beings, have antioxidant defense systems that protect against oxidative damage. However, these natural antioxidant mechanisms can be insufficient, and so extra intakes of antioxidant compounds has become important. Also, synthetic antioxidants for food preserving purposes have some side effects.16 Therefore, researches in the determination of natural sources of antioxidants and the antioxidant from plants are important.17
Phenolic compounds display good antioxidant activity in vitro and play a significant role in the protection of vegetable and plants against UV radiation, pathogens, and predators.18 Phenolic compounds are crucial plant constituents because of their ability to scavenge radicals and active oxygen species such as singlet oxygen, free radicals, and hydroxyl radicals.19,20 Bio-products have served as a big source of medicines since antique times and an important for natural drugs. In recent years, more interest in obtaining biologically active compounds from natural sources has been searched. Therefore, broad fields of bioactivity assays and other methods have been developed.8,21
Since the high performance liquid chromatography/diode array detector (HPLC/DAD) identification of phenolic components and the determination of their concentrations have already been assessed from the same extracts.22 The chemical composition was also given to compare in this article in order.
In this study, juniper and olive woods were preferred due to naturally durable species and the common usage areas. This study is referred to first study about DNA protective, antimicrobial and enzyme inhibitor activities. This study makes a significant contribution to research on biological activities of these valuable trees. In addition, this research serves as a basis for future studies and provides information for understanding biological functions of wood extracts of olive (O. europaea) and juniper (J. foetidissima) woods.
2. Materials and methods
2.1 Preparation of wood samples
Olive (O. europaea var. sylvestris Mill.) and juniper (J. foetidissima Willd.) wood samples were obtained from the Balıkesir province of Turkey in June of 2012, according to the TAPPI standard.23 Trees of each species were randomly selected at different locations. Wood samples were prepared from freshly cutted wood. Samples were left under sterile and 23 ± 2 °C, 30 ± 10% low humidity conditions for 3 months. The conditioned and dried wood samples were chopped into small parts with a special knife and powdered with a hammer mill for sapwood and heartwood. The wood powder (particle size between 0.05 and 0.4 mm) was stored in closed glass jars at room temperature.2.2 Extraction
The extraction was carried out using Soxhlet extractors in accordance with TAPPI standard.24 Wood samples (10 g) were extracted with methanol (150 mL) for 6 h. Then, the methanol solvent was evaporated and dried extract samples were kept at −18 °C until further experiments. Methanol has good polarity and high extraction yield. Hence this solvent is used favorably to extract polar components including phenolic compounds and flavonoids.25 This case was summarized in Table 1. Thus, methanol was selected as an only solvent in this study. Similarly, methanol was used by many researchers as an only solvent for extraction of wood or wood products.26–29| Solvents | Dielectric constant | Polarity | pKa | Yield (%) | References |
|---|---|---|---|---|---|
| Methanol | 32.6 | 5.2 | 16 | 18.6 | Šliumpaitė et al.30 |
| 7.32–11.27 | Tumen et al.31 | ||||
| 13.4 | Mori-Yasumoto et al.32 | ||||
| 1.97–19.88 | Bremaud et al.33 | ||||
| 1.63–17.50 | Kawamura et al.34 | ||||
| 34.18 | Onivogui et al.35 | ||||
| 20.88 | Brusotti et al.36 | ||||
| Ethanol | 22.4 | 5.1 | 16 | 6.24–10.34 | Tumen et al.31 |
| 14.46 | Rajesh et al.37 | ||||
| Acetone | 20.7 | 5.1 | 19.3 | 5.6 | Šliumpaitė et al.30 |
| 8.8 | Mori-Yasumoto et al.32 | ||||
| Ethyl acetate | 6.02 | 4.4 | 25 | 1.72 | Brusotti et al.36 |
| Chloroform | 4.81 | 4.1 | 15.5 | 1.33 | Rajesh et al.37 |
2.3 Microorganism strains
Gram positive (Listeria monocytogenes ATCC 7644, Enterococcus faecium, Staphylococcus aureus ATCC 25923, Enterococcus durans) and Gram negative (Enterobacter aerogenes ATCC 13048, Salmonella kentucky, Salmonella typhimurium SL 1344, Escherichia coli) bacteria were selected to test the antibacterial activity of wood extracts. Candida albicans ATCC 26555 was used to test the antifungal activity of wood extracts. Standard strains were provided from Department of Biology in Kastamonu University. Other strains were isolated from food and identified at Department of Biology in Ankara University.2.4 Antioxidant capacity assays
2.5 Reducing power
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v/v). Then, the sample absorbance was read at 593 nm after 30 min incubation at room temperature. FRAP activity was expressed as milligrams of trolox equivalents (mg TE per g extract).
1 (v/v/v). Then, the sample absorbance was read at 593 nm after 30 min incubation at room temperature. FRAP activity was expressed as milligrams of trolox equivalents (mg TE per g extract).2.6 Phosphomolybdenum method
The total antioxidant activity of the samples was evaluated by phosphomolybdenum method according to Berk et al.41 with slight modification. Sample solution (0.3 mL) was combined with 3 mL of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The sample absorbance was read at 695 nm after 90 min incubation at 95 °C. The total antioxidant capacity was expressed as millimoles of trolox equivalents (mmol TE per g extract).2.7 Metal chelating activity on ferrous ions
The metal chelating activity on ferrous ions was determined by the method described by Aktumsek et al.40 Briefly, sample solution (2 mL) was added to FeCl2 solution (0.05 mL, 2 mM). The reaction was initiated by the addition of 5 mM ferrozine (0.2 mL). Similarly, a blank was prepared by adding sample solution (2 mL) to FeCl2 solution (0.05 mL, 2 mM) and water (0.2 mL) without ferrozine. Then, the sample and blank absorbances were read at 562 nm after 10 min incubation at room temperature. The absorbance of the blank was subtracted from that of the sample. The metal chelating activity was expressed as milligrams of EDTA (disodium edetate) equivalents (mg EDTAE per g extract).2.8 Antibacterial activity
Antibacterial activity was carried out by disk-diffusion method based on Andrews.42 The nutrient media was poured into 100 mm sterile Petridish to give an average depth of 4.0 ± 0.5 mm.43,44 Dried wood extracts were dissolved in methanol to a final concentration of 20 mg mL−1. A total of 0.6 mg, 1 mg, 1.6 mg of olive wood extracts and 2 mg, 3 mg, 4 mg juniper wood extracts were applied on sterile 6 mm in diameter paper disks.45,46 Finally, disks were left to dry overnight at 30 °C in sterile conditions for evaporation of solvent residual.46,47A single colony of the bacteria strain was taken and inoculated into 20 mL of nutrient broth medium. This medium was incubated at 37 °C ± 2 °C for 24 h. Then, added to bacterial suspension to prepare a bacterial concentration into 0.9% sterile saline solution until the visible turbidity was equal to 0.5 McFarland standards having 108 cfu mL−1.48 Prepared bacterial suspension was spread on Petridish. Inoculated plates were then left to dry for 5 min at room temperature before applying the disks. Control disk (no extracts) and disks filled with extracts were placed on the surface of inoculated same Petridish (Mueller-Hinton). The inoculated plates were incubated at 37 °C for 24 h. The antibacterial activity was evaluated by measuring the zone of inhibition including disk against the test organisms.
2.9 DNA protection test
The DNA protection assay was performed using pUC 19 plasmid DNA (pDNA). The reaction mixture was prepared with 13.5 μL of distilled water, 0.5 μL of Fenton's reagent (30 mM H2O2, 50 mM ascorbic acid, and 80 mM FeCI3), 5 μL wood extracts at two concentration (5 μg μL−1, 10 μg μL−1) and 1 μL of pDNA (275 μg μL−1). Positive control was composed of 18.5 μL of distilled water, 0.5 μL of Fenton's reagent and 1 μL of pDNA. Negative control contains only 19 μL of distilled water and 1 μL of pDNA. After incubation for 30 min at 37 °C, 4 μL loading dye (Thermo Scientific, USA) was added to the all mixtures. The DNA mixtures were run on 1% agarose gel and then visualized under ultraviolet light cabin. The test was repeated at three times and band density was determined by the gel image analysis software (Quantum, Vision-Capt., Vilber Lourmat SAS, France).2.10 Enzyme inhibitory activity
Sample solution (25 μL) was mixed with tyrosinase solution (40 μL) and phosphate buffer (100 μL, pH 6.8) in a 96-well microplate and incubated for 15 min at 25 °C. The reaction was then initiated with the addition of L-DOPA (40 μL). Similarly, a blank was prepared by adding sample solution to all reaction reagents without enzyme (tyrosinase) solution. The sample and blank absorbances were read at 492 nm after a 10 min incubation at 25 °C. The absorbance of the blank was subtracted from that of the sample and the tyrosinase inhibitory activity was expressed as equivalents of kojic acid (mg KAEs per g extract).
2.11 Statistical analysis
For all the experiments and assays were carried out in triplicate. The results are expressed as mean values and standard deviation (SD). The differences between the different extracts were analyzed using one-way analysis of variance (ANOVA) with SPSS v. 14.0 program followed by Tukey's honestly significant difference post hoc test with α = 0.05.3. Results and discussion
3.1 The earlier HPLC/DAD fingerprint results of the extracts
HPLC/DAD identification and quantification of polyphenolic compounds of the tested extracts have already been carried out by the authors.22 Fig. 1 and 2 depicted the chromatograms of these extracts. Apparently, these extracts contained different levels of these components. For example, oleuropein was identified as major component in olive extracts while catechin was the dominant component in the juniper extracts (Table 2).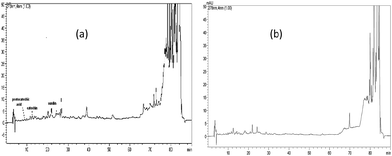 | ||
| Fig. 1 Juniper heartwood (a) and sapwood (b) HPLC chromatogram.22 | ||
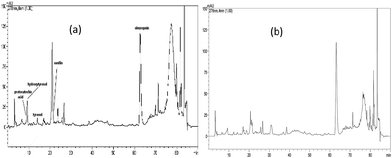 | ||
| Fig. 2 Olive heartwood (a) and sapwood (b) HPLC chromatogram.22 | ||
| Phenolic components | Juniper sapwood | Juniper heartwood | Olive sapwood | Olive heartwood |
|---|---|---|---|---|
| a nd: not determined. | ||||
| Protocatechic acid | 0.2 | 0.3 | 1.4 | 1.5 |
| Catechin | 3.7 | 3.7 | nd | nd |
| Vanillin | 0.4 | 0.5 | 1.5 | 0.9 |
| p-Coumaric acid | nd | nd | 0.4 | nd |
| Benzoic acid | nd | nd | 9.9 | nd |
| Hydroxytyrosol | nd | nd | 8.2 | 29.5 |
| Tyrosol | nd | nd | 3.3 | 10.7 |
| Oleuropein | nd | nd | 703.2 | 746.0 |
3.2 Antioxidant capacity
The antioxidant capacities of wood extracts in this study were assayed with five different assays including DPPH scavenging, FRAP, CUPRAC, metal chelating and phosphomolybdenum assays, because the antioxidant capacity cannot be fully described by a single method.Free radical scavenging activities of wood extracts were evaluated by DPPH assay. The method is based on the reduction of alcoholic DPPH solution in the presence of antioxidant compounds, due to the formation of the non-radical form DPPH-H by the reaction. The reaction was reduced purple DPPH radical to yellow DPPH-H. In the ranking of the radical scavenging activity was obtained by this method, the O. europaea heartwood showed the highest activity (116.05 mg TEs per g extract), followed by O. europaea var. slyvestris sapwood (82.82 mg TEs per g extract), J. foetidissima sapwood (69.64 mg TEs per g extract) and J. foetidissima heartwood (65.22 mg TEs per g extract). Olive wood extracts contain important constituents including hydroxytyrosol, tyrosol and oleuropein.8,22,50 These constituents possess several biological activities such as radical scavenger.8 In this direction, the superior free radical scavenging activities of O. europaea var. slyvestris extracts may be caused due to the presence of these compounds.
The reducing power of a compound may serve as a significant indicator of its potential antioxidant activity. For the measurements of the reductive ability, we investigated the Fe3+–Fe2+ and Cu2+ and Cu+ transformation in the presence of wood extracts. As shown in Table 3, the wood extracts showed very different reducing powers (p < 0.05). Similar to the results obtained from DPPH assay, the O. europaea is heartwood extract exhibited the strongest reducing activity in both FRAP (105.23 mg TEs per g extract) and CUPRAC (192.91 mg TEs per g extract), compared to those of other extracts. J. foetidissima sapwood had the lowest reducing potentials in these assays. In accordance with our results, some authors were observed a positive correlation between antioxidant activities and reducing power of certain plant extracts.51,52
| Samples | Chelating effectb (mg EDTAEs per g extract) | Phosphomolybdenumc (mmol TEs per g extract) | CUPRACc (mg TEs per g extract) | FRAPc (mg TEs per g extract) | DPPHc (mg TEs per g extract) | |
|---|---|---|---|---|---|---|
| a In same column marked with different letters indicate significant difference (p < 0.05).b EDTAEs. disodium edetate equivalents.c TEs. trolox equivalents. | ||||||
| J. foetidissima | Sapwood | 5.76 ± 0.17b,c | 0.99 ± 0.08c | 50.95 ± 2.61 | 33.02 ± 0.64 | 69.64 ± 0.74c |
| Heartwood | 21.98 ± 1.15a | 3.02 ± 0.17b | 88.65 ± 0.78c | 61.44 ± 0.55c | 65.22 ± 1.70c | |
| O. europaea | Sapwood | 1.68 ± 0.31 | 3.01 ± 0.08b | 151.78 ± 5.37b | 97.35 ± 1.23b | 82.82 ± 0.08b |
| Heartwood | 4.69 ± 1.36c | 3.69 ± 0.15a | 192.91 ± 4.42a | 105.23 ± 0.93a | 116.05 ± 1.90a | |
Also, our results are consistent with Conde et al.53 who indicated that the O. europaea pruning showed significantly ferric reducing activity in FRAP assay. Phosphomolybenum assay is based on the reduction of Mo(VI) to Mo(V) by the antioxidants and subsequent formation of green complex (phosphate/Mo(V)) which is measured spectrophotometrically at 695 nm. In the assay, heartwood extracts performed higher activities than sapwood extracts. The lowest activity was observed in J. foetidissima sapwood extract. Apparently, the study reveals that the antioxidant activity of J. foetidissima heartwood extract was about 4-fold higher than that of sapwood extract.
Transition metal ions joined the production of free radicals and thus, are considered as effective pro-oxidants. The purpose of the test of ferrous ion chelating activity was to determine the capacity of the wood extracts to bind the ferrous ion catalyzing oxidation. As can be seen from Table 3, J. foetidissima heartwood extract had the highest ferrous ion chelating capacity (21.98 mg EDTAs per g extract) followed by juniper sapwood (5.76 mg EDTAs per g extract), olive heartwood (4.69 mg EDTAs per g extract) and olive sapwood (1.68 mg EDTAs per g extract) extracts. These results revealed significant differences in the ferrous ion chelating activity of wood extracts tested (p < 0.05). Interestingly, olive wood extracts significantly have stronger scavenging activities against free radical and reducing power abilities. They also exhibited lower chelating effects. Similarly, there are contradictory reports in the literature regarding between metal chelating capacity and other antioxidant assays.54,55 This situation can be explained by the presence of many chelators in phytochemicals, including non-phenolics, polysaccharides, peptides and proteins.
To the best of our knowledge, antioxidant capacities of different parts of both Juniperus and Olea species were previously investigated.3,56–59
However, no scientific studies are reported on the antioxidant properties of wood extracts obtained from J. foetidissima and O. europaea var. slyvestris before. Thus, the antioxidant capacities of these wood extracts were reported for the first time in the present study.
3.3 Antibacterial activity
The heartwood and sapwood extracts from juniper and olive were examined against 8 bacteria and 1 fungus strains based on of disk-diffusion method. The juniper wood extracts at a concentration of 2 mg, 3 mg and 4 mg per disk and olive wood extracts at a concentration of 0.6 mg, 1 mg and 1.6 mg per disk were used for antimicrobial activity. The wood extracts showed diverse degrees of antibacterial activities depending on bacterial strains, extracts samples and extract amounts (Table 4).| Microorganisms | Olive sapwood | Olive heartwood | Juniper heartwood | Juniper sapwood | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.6 mg | 1 mg | 1.6 mg | 0.6 mg | 1 mg | 1.6 mg | 2 mg | 3 mg | 4 mg | 2 mg | 3 mg | 4 mg | |
| a na. not active. | ||||||||||||
| Listeria monocytogenes | 16 | 25 | 26 | 9 | 10 | 10 | 8 | 9 | 10 | na | na | 9 |
| Enterococcus faecium | 20 | 23 | 26 | na | na | 10 | 16 | 19 | 21 | 8 | 10 | 20 |
| Staphylococcus aureus | 11 | 15 | 16 | 10 | 12 | 12 | 13 | 17 | 21 | 10 | 11 | 16 |
| Enterococcus durans | na | na | 8 | na | 8 | 13 | 9 | 11 | 12 | na | na | 8 |
| Enterobacter aerogenes | 9 | 11 | 13 | na | na | na | na | 7 | 8 | 6 | 6 | 7 |
| Salmonella kentucky | na | na | 8 | na | na | na | na | na | 8 | 6 | 6 | 8 |
| Salmonella typhimurium | 12 | 12 | 13 | na | na | na | 6 | 10 | 12 | na | na | 11 |
| Escherichia coli | na | 6 | 8 | 8 | 8 | 8 | 8 | 9 | 10 | na | 7 | 8 |
| Candida albicans | na | na | 7 | na | na | 7 | 8 | 8 | 9 | na | na | 9 |
The results revealed that olive sapwood and both wood extracts from juniper had a strong antibacterial activity against almost all test bacteria strains. However, olive heartwood extracts did not show antibacterial activity on Enterobacter aerogenes, Salmonella kentucky and Salmonella typhimurium. The best antibacterial activity was observed against Listeria monocytogenes with inhibition zone of 26, 25 and 16 mm using olive sapwood extracts 1.6, 1 and 0.6 mg, respectively (Table 4).
Listeria monocytogenes is one of the most virulent food-borne pathogens some of clinical infections resulting in death.60 Enterococcus faecium is a highly drug resistant human pathogen that causes infections.61 Staphylococcus aureus is one of the most successful and flexible human pathogens.62 Salmonella typhimurium is a pathogenic bacteria. Its toxicity is due to an outer membrane which protects the bacteria from the environment factors.63 Escherichia coli is a concern to public health on a wide scale64 and it is contaminated a wide variety of food products.65 Candida albicans is the primary cause of fungal infections in neutropenic and solid-organ transplant patients, and is also implicated in oral candidiasis cases found in HIV patients.66 Most of the tested bacteria and fungi strains are pathogenic for human or food. Some of them have resistance against to drugs. Therefore, we have selected and tested these organisms to see effect and power of natural wood extracts against to these strains.
We found that Gram-positive (GP) bacteria strains seemed to be more susceptible to the extracts than the Gram-negative (GN) strains. These findings are consistent with those of Boulekbache-Makhlouf67 and Trigui et al.68 who reported that GP bacteria was more sensitive to the extracts than GN ones. GN bacteria strains (S. aureus) are generally less susceptible to plant extracts than the GP bacteria, because they have an outer membrane which provides a barrier against biomolecules.69
It was found that olive sapwood and both juniper wood extracts showed high antibacterial activity with inhibition zone from 7 to 26 mm and 6–21 mm, respectively. These results agree with the findings of other studies, in which ethyl acetate extracts of Thymelaea hirsuta showed high antibacterial activity with inhibition zone varied from 9 to 15 mm against various bacteria strains Staphylococcus aureus, Listeria monocytogenes.68 Olive sapwood (1.6 mg), olive heartwood (1.6 mg), juniper heartwood (2,3,4 mg) and juniper sapwood (4 mg) was also showed antifungal activity against Candida albicans.
In our previous study (Ateş et al.22), chemical contents and antifungal activities of these sapwood and heartwood methanol extracts from olive and juniper were reported. We found different phenolic compounds from olive heartwood and sapwood including oleuropein, hydroxytyrosol, tyrosol, vanillin, protocatechic acid, benzoic acid, and p-coumaric acid. The total phenolic content of juniper heartwood was greater than other extracts. Also, we observed that olive and juniper wood extracts showed good antifungal activities.
Previous studies have shown that oleuropein, hydroxytyrosol, tyrosol, benzoic acid, p-coumaric acid, protocatechic and catechin exhibit good antifungal, antioxidant and antibacterial activity.9,70–74 The observed findings in this study mirror those of the previous studies that have examined effects of phenolic compounds on antimicrobial activities. In this study, the good antibacterial activities of olive and juniper wood extracts may be caused due to the presence of these phenolic compounds. As a result, methanol extracts of wood samples from olive and juniper could be used as one of the sources of natural antibacterial agents.
3.4 DNA protection test
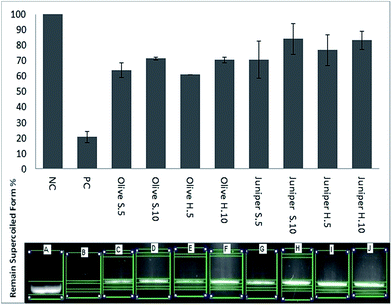
The DNA protection test was carried out to confirm the protection ability of different wood extracts against hydroxyl radical damage generated by Fenton Reagent's. Fig. 3 shows the DNA damage protection activity of olive and juniper wood extracts with two different concentrations (5 and 10 mg mL−1).Juniper sapwood (Juniper S.10 mg mL−1) had the highest DNA protection activity (84%), followed by Juniper H.10 (83%) and Juniper H.5 (77%). About 71% remained supercoiled form was observed with Olive S.10 and Olive H.10. Although all wood extracts indicated DNA protection ability, the lowest activity was obtained from Olive H.5 and Olive S.5. All extract samples concentration of 10 mg mL−1 shows more protective effects against hydroxyl radicals than 5 mg mL−1. Although olive oil has a protective effect against DNA damage,13 this study is the first report on DNA protection activity using heartwood and sapwood of olive tree.
Reactive hydroxyl radicals are formed in all biological systems and can damage almost every molecule in living cells and human body.75 Also, they join nucleotides in DNA and cause strand degradation which result in mutagenesis. For this reasons, natural compounds which have antioxidants and hydroxyl radical scavenging activities have become more important than before.76
3.5 Enzyme inhibitory activities
Recently, inhibition of key enzymes related to pathology in most public health problems including Alzheimer's and Diabetes mellitus play vital important role. For example, acetylcholinesterase (AChE) catalyzes the hydrolysis of acetylcholine (ACh), which is important to continue neural transmission in the synaptic left. In Alzheimer's pathology, the lack of ACh was observed and this case gives rise to so-called cholinergic hypothesis. In this point, AChE inhibitors are considered as the most useful relieving strategy in the treatment of Alzheimer's disease.77,78 As to Diabetes mellitus, two enzymes (α-amylase and α-glucosidase), which catalyze hydrolysis of starch, are increased blood glucose. In this direction, the inhibition of these enzymes can be an important strategy in the management of blood glucose level linked to Diabetes mellitus.79 Tyrosinase is known as a key enzyme in melanin synthesis. Thus, the blocking of melanin synthesis by tyrosinase inhibitors are considered as an effective treatment strategy for pigmentation disorders.80 To this end, several enzyme inhibitors (galantamine, tacrine and rivastigmine for Alzheimer, acarbose and viglibose for Diabetes mellitus and kojic acid for skin diseases) were synthetically developed for pharmacology industry, but they may possess some side effects such as gastrointestinal disturbances and liver damage.81–83 Therefore, there is great attention in exploring new and safe inhibitors from natural sources.The enzyme inhibitory activities were measured with spectrophotometric methods and the results were illustrated in Table 5. Similar to antioxidant assay results, Olive sapwood extracts displayed the strongest enzyme inhibitory activities against AChE (2.47 mg GALAEs per g extract) and BChE (5.95 mg GALAEs per g extract). J. foetidissima heartwood extract showed no ability to inhibit BChE. Interestingly, J. foetidissima heartwood extract had the highest anti-amylase (1.25 mmol ACEs per g extract), anti-glucosidase (41.03 mmol ACEs per g extract) and anti-tyrosinase (58.05 KAEs per g extract) effect. The lowest anti-tyrosinase activity was observed in O. europaea heartwood extract. The differences observed in the enzyme inhibition can be explained with complex nature of phytochemicals and different chemical profile of the extracts tested. In a study on anti-diabetic potential leaves of J. oxycedrus subsp. oxycedrus were examined using in vivo assays.
| Samples | Acetylcholinesteraseb (mg GALAEs per g extract) | Butyrylcholinesteraseb (mg GALAEs per g extract) | α-Amylasec (mmol ACEs per g extract) | α-Glucosidasec (mmol ACEs per g extract) | Tyrosinased (mg KAEs per g extract) | |
|---|---|---|---|---|---|---|
| a In same column marked with different letters indicate significant difference (p < 0.05); na. not active.b GALAEs. galanthamine equivalents.c ACEs. acarbose equivalents.d KAEs. kojic acid equivalent. | ||||||
| J. foetidissima | Sapwood | 2.15 ± 0.03b | 5.10 ± 0.18c | 0.83 ± 0.01b | 12.03 ± 3.84b | 56.29 ± 0.94a |
| Heartwood | 1.88 ± 0.08c | na | 1.25 ± 0.11a | 41.03 ± 1.29a | 58.05 ± 2.32a | |
| O. europaea | Sapwood | 2.47 ± 0.10a | 5.95 ± 0.03a | 0.60 ± 0.01c | 16.10 ± 0.66b | 30.65 ± 3.66b |
| Heartwood | 2.38 ± 0.05a | 5.52 ± 0.17b | 0.62 ± 0.03c | 15.75 ± 0.64b | 24.58 ± 2.93b | |
It showed a potent antidiabetic effect in these tests, which is in accordance with our data.84 Significant anticholinesterase activities of five Juniperus species from Turkey were studied by Orhan et al.3 In the other study, Jazayeri et al.85 reported that fruit of J. sabina had an anticholinesterase effect. Anti-tyrosinase and anti-diabetic abilities of Olea leaf extracts or olive were confirmed by several researchers.86–88 A close relationship between the enzyme inhibitory potentials and chemical constituents (especially oleuropein, hydroxytyrosol and tyrosol) of olive leaf extracts was observed in these studies. However, to the best of our knowledge, this study is the first report on enzyme inhibitory abilities of the wood extracts studied.
4. Conclusion
To sum up, the wood extracts have notable antioxidant and enzyme inhibitory potentials. The extracts also showed good antibacterial activity against most of bacteria strains, especially GP strains including L. monocytogenes, E. faecium, S. aureus which have not outer membrane. However, GN bacteria have an outer membrane, which is quite impermeable to lipophilic molecules such as hydrophobic antibiotics, detergents and hydrophobic dyes.89 Salmonella typhimurium is a pathogenic Gram-negative bacteria predominately found in the intestinal lumen. Its toxicity is due to an outer membrane consisting largely of lipopolysaccharides (LPS) which protect the bacteria from the environment.90 The outer membrane also acts as a selective barrier against to hydrophilic molecules. Scalbert,91 reported that high molecular weight tannins are toxic to S. aureus but not to E. coli. These extracts also inhibited hydroxyl radicals which degraded DNA. When the both sapwood and heartwood extracts concentration increased in DNA damage protection test, the protection activity of the wood extracts increased. These results suggested that the wood extracts from juniper and olive can be exploited as a potential source of natural agents for food and pharmacology industries.Acknowledgements
This study was supported by the Kastamonu University Scientific Projects Research Office (Project Number KÜBAP-01/2013-37).Notes and references
- R. P. Adams, Junipers of the World: The genus Juniperus, Trafford Publishing Co., Vancouver, 2008 Search PubMed.
- P. H. Davis, The flora of Turkey and East Aegean Islands, Edinburg, 1997 Search PubMed.
- N. Orhan, I. E. Orhan and F. Ergun, Food Chem. Toxicol., 2011, 49, 2305–2312 CrossRef CAS PubMed.
- I. Karaman, F. Şahin, M. Güllüce, H. Öǧütçü, M. Şengül and A. Adıgüzel, J. Ethnopharmacol., 2003, 85, 231–235 CrossRef CAS.
- M. M. Lesjak, I. N. Beara, D. Z. Orčić, J. D. Ristić, G. T. Anačkov, B. N. Božin and N. M. Mimica-Dukić, LWT--Food Sci. Technol., 2013, 53, 530–539 CrossRef CAS PubMed.
- A. Mrabet, M. Rejili, B. Lachiheb, P. Toivonen, N. Chaira and A. Ferchichi, Ann. Microbiol., 2008, 58, 453–459 CrossRef CAS.
- A. Y. Leung and S. Foster, Encyclopedia of common natural ingredients used in food, drugs, and cosmetics, John Wiley & Sons, Inc., 1996 Search PubMed.
- M. Pérez-Bonilla, S. Salido, T. A. van Beek, P. J. Linares-Palomino, J. Altarejos, M. Nogueras and A. Sánchez, J. Chromatogr. A, 2006, 1112, 311–318 CrossRef PubMed.
- G. Bisignano, A. Tomaino, R. L. Cascio, G. Crisafi, N. Uccella and A. Saija, J. Pharm. Pharmacol., 1999, 51, 971–974 CrossRef CAS.
- O. Benavente-Garcıa, J. Castillo, J. Lorente, A. Ortuno and J. Del Rio, Food Chem., 2000, 68, 457–462 CrossRef.
- H. K. Hamdi and R. Castellon, Biochem. Biophys. Res. Commun., 2005, 334, 769–778 CrossRef CAS PubMed.
- V. Micol, N. Caturla, L. Pérez-Fons, V. Más, L. Pérez and A. Estepa, Antiviral Res., 2005, 66, 129–136 CrossRef CAS PubMed.
- Ö. Erol, N. Arda and G. Erdem, Food Chem. Toxicol., 2012, 50, 3475–3479 CrossRef PubMed.
- O. I. Aruoma and S. L. Cuppett, Antioxidant methodology: in vivo and in vitro concepts, The American Oil Chemists Society, 1997 Search PubMed.
- B. Halliwell and J. M. C. Gutteridge, Free Radicals in Biology and Medicine, Calenderon Press, Oxford, UK, 1989 Search PubMed.
- N. Ito, S. Fukushima, A. Haqlwara, M. Shibata and T. Ogiso, J. Natl. Cancer Inst., 1983, 70, 343–352 CAS.
- S. Kordali, R. Kotan, A. Mavi, A. Cakir, A. Ala and A. Yildirim, J. Agric. Food Chem., 2005, 53, 9452–9458 CrossRef CAS PubMed.
- D. Strack, in Plant Biochemistry, ed. P. M. D. B. Harborne, Academic Press, London, 1997, pp. 387–416 Search PubMed.
- C. Hall and S. Cuppett, Antioxidant methodology in vivo and in vitro concepts, 1997, pp. 2–29 Search PubMed.
- A. Güder and H. Korkmaz, Iran. J. Pharm. Res., 2012, 11, 913 Search PubMed.
- A. Moure, J. M. Cruz, D. Franco, J. M. Domínguez, J. Sineiro, H. Domínguez, M. A. J. Núñez and J. C. Parajó, Food Chem., 2001, 72, 145–171 CrossRef CAS.
- S. Ateş, M. Gür, O. E. Özkan, M. Akça, Ç. Olgun and A. Güder, BioResources, 2015, 10, 2433–2443 CrossRef.
- TAPPI, Sampling and preparing wood for analysis, TAPPI Press, Atlanta, GA, USA, 1985 Search PubMed.
- TAPPI, Solvent extractives of wood and pulp, TAPPI Press, Atlanta, GA, USA, 1988 Search PubMed.
- M. N. Alam, N. J. Bristi and M. Rafiquzzaman, Saudi Pharm. J., 2013, 21, 143–152 CrossRef PubMed.
- S. Jayaraman and R. Mohamed, Turk. J. Agric. For., 2015, 39, 163–173 CrossRef.
- S. Duangsrisai, K. Choowongkomon, L. J. Bessa, P. M. Costa, N. Amat and A. Kijjoa, Molecules, 2014, 19, 19923–19934 CrossRef PubMed.
- F. Kawamura, S. F. M. Ramle, O. Sulaiman, R. Hashim and S. Ohara, Eur. J. Wood Wood Prod., 2011, 69, 207–212 CrossRef CAS.
- M.-L. Xu, L. Wang, J.-H. Hu, S. K. Lee and M.-H. Wang, Food Sci. Biotechnol., 2010, 19, 677–682 CrossRef CAS.
- I. Šliumpaitė, P. Venskutonis, M. Murkovic and O. Ragažinskienė, Ind. Crops Prod., 2013, 50, 715–722 CrossRef PubMed.
- I. Tumen, F. J. Eller, C. A. Clausen and J. A. Teel, BioResources, 2012, 8, 12–20 CrossRef.
- K. Mori-Yasumoto, R. Izumoto, H. Fuchino, T. Ooi, Y. Agatsuma, T. Kusumi, M. Satake and S. Sekita, Bioorg. Med. Chem., 2012, 20, 5215–5219 CrossRef CAS PubMed.
- I. Brémaud, N. Amusant, K. Minato, J. Gril and B. Thibaut, Wood Sci. Technol., 2011, 45, 461–472 CrossRef.
- F. Kawamura, A. Mahamud, O. Sulaiman and R. Hashim, J. Trop. Forest Sci., 2010, 170–174 Search PubMed.
- G. Onivogui, M. Diaby, X. Chen, H. Zhang and Y. Song, J. Ethnopharmacol., 2015, 168, 287–290 CrossRef CAS PubMed.
- G. Brusotti, S. Tosi, A. Tava, A. M. Picco, P. Grisoli, I. Cesari and G. Caccialanza, Ind. Crops Prod., 2013, 43, 612–616 CrossRef CAS PubMed.
- K. Rajesh, H. Manjunatha, V. Krishna and B. K. Swamy, Ind. Crops Prod., 2013, 47, 186–198 CrossRef CAS PubMed.
- C. Sarikurkcu, Afr. J. Biotechnol., 2013, 10, 831–839 Search PubMed.
- G. Zengin, C. Sarikurkcu, A. Aktumsek, R. Ceylan and O. Ceylan, Ind. Crops Prod., 2014, 53, 244–251 CrossRef CAS PubMed.
- A. Aktumsek, G. Zengin, G. O. Guler, Y. S. Cakmak and A. Duran, Food Chem. Toxicol., 2013, 55, 290–296 CrossRef CAS PubMed.
- S. Berk, B. Tepe, S. Arslan and C. Sarikurkcu, Afr. J. Biotechnol., 2013, 10, 8902–8908 Search PubMed.
- J. M. Andrews, J. Antimicrob. Chemother., 2005, 56, 60–76 CrossRef CAS PubMed.
- E. Altuner and B. Çetin, Fen Bilimleri Enst. Derg., 2009, 2, 85–92 Search PubMed.
- E. M. Altuner and I. Akata, SAU Fen. Bil. Der., 2010, 14, 45–49 Search PubMed.
- A. M. Mahasneh and A. A. El-Oqlah, J. Ethnopharmacol., 1999, 64, 271–276 CrossRef CAS.
- S. Silici and A. Koc, Lett. Appl. Microbiol., 2006, 43, 318–324 CrossRef CAS PubMed.
- E. Altuner, B. Çetin and C. Çökmüş, Kastamonu Üniversitesi Orman Fakültesi Dergisi, 2010, 10, 111–116 Search PubMed.
- E. M. Altuner, I. Akata and K. Canli, Fresenius Environ. Bull., 2012, 21, 3407–3410 CAS.
- I. E. Orhan, F. S. Senol, A. R. Gulpinar, N. Sekeroglu, M. Kartal and B. Sener, Food Chem., 2012, 130, 882–888 CrossRef CAS PubMed.
- J. Altarejos, S. Salido, M. Pérez-Bonilla, P. J. Linares-Palomino, T. A. van Beek, M. Nogueras and A. Sánchez, Fitoterapia, 2005, 76, 348–351 CrossRef PubMed.
- G.-L. Chen, S.-G. Chen, Y.-Y. Zhao, C.-X. Luo, J. Li and Y.-Q. Gao, Ind. Crops Prod., 2014, 57, 150–157 CrossRef CAS PubMed.
- A. A. Hatamnia, N. Abbaspour and R. Darvishzadeh, Food Chem., 2014, 145, 306–311 CrossRef CAS PubMed.
- E. Conde, C. Cara, A. Moure, E. Ruiz, E. Castro and H. Domínguez, Food Chem., 2009, 114, 806–812 CrossRef CAS PubMed.
- K. S. Farvin and C. Jacobsen, Food Chem., 2013, 138, 1670–1681 CrossRef PubMed.
- R. Fu, Y. Zhang, Y. Guo, F. Liu and F. Chen, Ind. Crops Prod., 2014, 58, 265–270 CrossRef CAS PubMed.
- N. Orhan, M. Aslan, M. Pekcan, D. D. Orhan, E. Bedir and F. Ergun, J. Ethnopharmacol., 2012, 139, 110–118 CrossRef CAS PubMed.
- M. F. Taviano, A. Marino, A. Trovato, V. Bellinghieri, A. Melchini, P. Dugo, F. Cacciola, P. Donato, L. Mondello and A. Güvenç, Food Chem. Toxicol., 2013, 58, 22–29 CrossRef CAS PubMed.
- S. Silva, L. Gomes, F. Leitao, A. Coelho and L. V. Boas, Food Sci. Technol. Int., 2006, 12, 385–395 CrossRef CAS PubMed.
- A. Sousa, S. Casal, R. Malheiro, H. Lamas, A. Bento and J. A. Pereira, LWT--Food Sci. Technol., 2015, 60, 22–28 CrossRef CAS PubMed.
- V. Ramaswamy, V. M. Cresence, J. S. Rejitha, M. U. Lekshmi, K. Dharsana, S. P. Prasad and H. M. Vijila, J. Microbiol., Immunol. Infect., 2007, 40, 4 CAS.
- M. M. Huycke, D. F. Sahm and M. S. Gilmore, Emerging Infect. Dis., 1998, 4, 239 CrossRef CAS PubMed.
- P. Sampathkumar, Mayo Clin. Proc., 2007, 82, 1463–1467 CrossRef.
- A. Tuin, A. Huizinga-van der Vlag, A.-M. M. van Loenen-Weemaes, D. K. Meijer and K. Poelstra, Am. J. Physiol.: Gastrointest. Liver Physiol., 2006, 290, G377–G385 CrossRef CAS PubMed.
- P. S. Mead and P. M. Griffin, Lancet, 1998, 352, 1207–1212 CrossRef CAS.
- R. L. Buchanan and M. P. Doyle, Food Technol., 1997, 51, 69–76 Search PubMed.
- J. L. Vincent, E. Anaissie, H. Bruining, W. Demajo, M. El-Ebiary, J. Haber, Y. Hiramatsu, G. Nitenberg, P. Nyström and D. Pittet, Intensive Care Med., 1998, 24, 206–216 CrossRef CAS.
- L. Boulekbache-Makhlouf, S. Slimani and K. Madani, Ind. Crops Prod., 2013, 41, 85–89 CrossRef CAS PubMed.
- M. Trigui, A. B. Hsouna, S. Tounsi and S. Jaoua, Ind. Crops Prod., 2013, 41, 150–157 CrossRef CAS PubMed.
- I. Chopra and J. Battista, Antibacterial agents: Basis of action, Wiley, New Jersey, 2001 Search PubMed.
- F. Daayf, R. Bel-Rhlid and R. R. Bélanger, J. Chem. Ecol., 1997, 23, 1517–1526 CrossRef CAS.
- B.-E. Amborabé, P. Fleurat-Lessard, J.-F. Chollet and G. Roblin, Plant Physiol. Biochem., 2002, 40, 1051–1060 CrossRef.
- M. Friedman, Mol. Nutr. Food Res., 2007, 51, 116–134 CAS.
- T. Yangui, S. Sayadi, A. Rhouma and A. Dhouib, J. Pestic. Sci., 2010, 83, 437–445 Search PubMed.
- X. Li, X. Wang, D. Chen and S. Chen, Funct. Foods Health Dis., 2011, 1, 232–244 CAS.
- P. Hochstein and A. S. Atallah, Mutat. Res., Fundam. Mol. Mech. Mutagen., 1988, 202, 363–375 CrossRef CAS.
- L. C. N. da Silva, C. A. da Silva, R. M. de Souza, A. J. Macedo, M. V. da Silva and M. T. dos Santos Correia, Food Chem. Toxicol., 2011, 49, 2222–2228 CrossRef PubMed.
- P. Williams, A. Sorribas and M.-J. R. Howes, Nat. Prod. Rep., 2011, 28, 48–77 RSC.
- S. Dall'Acqua, Bot.: Targets Ther., 2013, 3, 19–28 CrossRef.
- U. Etxeberria, A. L. de la Garza, J. Campión, J. A. Martínez and F. I. Milagro, Expert Opin. Ther. Targets, 2012, 16, 269–297 CrossRef CAS PubMed.
- Y.-J. Kim and H. Uyama, Cell. Mol. Life Sci., 2005, 62, 1707–1723 CrossRef CAS PubMed.
- E. A. Adewusi, N. Moodley and V. Steenkamp, S. Afr. J. Bot., 2011, 77, 638–644 CrossRef CAS PubMed.
- W. Yi, X. Wu, R. Cao, H. Song and L. Ma, Food Chem., 2009, 117, 381–386 CrossRef CAS PubMed.
- H.-Q. Dong, M. Li, F. Zhu, F.-L. Liu and J.-B. Huang, Food Chem., 2012, 130, 261–266 CrossRef CAS PubMed.
- N. Orhan, S. Hoçbaç, D. D. Orhan, M. Asian and F. Ergun, Iran. J. Basic Med. Sci., 2014, 17, 426 Search PubMed.
- S. B. Jazayeri, A. Amanlou, N. Ghanadian, P. Pasalar and M. Amanlou, Daru, J. Pharm. Sci., 2014, 22, 17 CrossRef PubMed.
- S. Bulotta, M. Celano, S. M. Lepore, T. Montalcini, A. Pujia and D. Russo, J. Transl. Med., 2014, 12, 219 CrossRef PubMed.
- E. Coni, R. Di Benedetto, M. Di Pasquale, R. Masella, D. Modesti, R. Mattei and E. Carlini, Lipids, 2000, 35, 45–54 CrossRef CAS.
- H. Poudyal, F. Campbell and L. Brown, J. Nutr., 2010, 140, 946–953 CrossRef CAS PubMed.
- P. W. Grosvenor, A. Supriono and D. O. Gray, J. Ethnopharmacol., 1995, 45, 97–111 CrossRef CAS.
- T. Annemarie, Am. J. Physiol.: Gastrointest. Liver Physiol., 2006, 290, G377–G385 CrossRef PubMed.
- A. Scalbert, Phytochemistry, 1991, 30, 3875–3883 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |