Superhydrophobic/superoleophilic magnetic polyurethane sponge for oil/water separation†
Shanhu Liu*,
Qingfeng Xu,
Sanjay S. Latthe,
Annaso B. Gurav and
Ruimin Xing*
Henan Key Laboratory of Polyoxometalate Chemistry, Institute of Molecular and Crystal Engineering, College of Chemistry and Chemical Engineering, Henan University, Kaifeng 475001, P. R. China. E-mail: shanhuliu@henu.edu.cn; rmxing@henu.edu.cn
First published on 3rd August 2015
Abstract
Oil/water separation is a worldwide challenge and addressing this challenge calls for the development of efficient absorbent materials. Here a superhydrophobic/superoleophilic magnetic polyurethane (PU) sponge was fabricated via the facile dopamine self-polymerization to anchor Fe3O4 nanoparticles onto the skeleton of the PU sponge, followed by the introduction of low-surface-energy hydrophobic molecules heptadecafluoro-1,1,2,2-tetrahydrodecyltrimethoxysilane (FAS-17) on the sponge surface to induce the superhydrophobic transformation. The magnetic PU sponge displays excellent superhydrophobicity and superoleophilicity, and more favorably possesses magnetic responsiveness and superior stability against corrosive solutions; it gave outstanding separation performance under magnetic actuation not only for floating oils on the water surface and heavy organic pollutants under water, but also in the more complex environments such as an acidic solution (pH = 1) and simulated seawater, suggesting great potential in practical oily wastewater treatment. The presented approach provides a facile and easily scalable solution for the design and construction of multifunctional magnetic absorbent materials with low costs for practical applications in the petro-chemical field.
1. Introduction
With the rapid industrial development, the frequent occurrence of oil spillages and indiscriminate discharge of organic solvents have caused severe environmental pollution and ecological damage. It has become a worldwide challenge to solve oil spill accidents and the increasing amount of industrial organic pollutants.1–5 Currently, there are several general strategies for removing oil from water, including controlled fuel burning, chemical dispersants and solidifiers;6–8 however, they would lead to additional environmental and ecological issues as well as high loss and non-portable operation. Recently, various functional materials based on stainless steel mesh, copper mesh, sponges and microfiltration membranes have been fabricated for oil/water separation.9–18 Among them, PU sponge is a kind of porous and hydrophilic polymer that possesses high porosity, light weight, good elasticity, and low-cost for large-scale production, which endows their promising applications in the fields of sorption, filtration, and separation. Strategies such as etching and chemical modification were often employed to enhance roughness for improving absorption capacity. For example, superhydrophobic and superoleophilic PU sponge was prepared by using chromic acid etching and fluoroalkylsilane chemical modification.19 CNTs-modified PU sponge was fabricated via an oxidative self-polymerization process of dopamine, followed by the reaction of hydrophilic polydopamine with hydrophobic octadecylamine.20 Magnetic foams were prepared via the pyrolysis of PU sponge grafted with metal acrylates at high temperature21 and triboelectric polytetrafluoroethylene particle deposition followed by casting magnetic solution,22 respectively. However, aggressive etching solutions, harsh conditions or sophisticated procedures were often required, which limits their commercial application because of the difficulty of large-scale fabrication and high cost. Furthermore, the stability towards corrosive liquids such as acidic and basic solutions was rarely presented, which is essential for their practical applications for oil/water separation owing to the complex oily wastewater environment. Therefore, a facile and easily scalable fabrication of super-wetting absorbent materials with low-cost, enhanced stability towards corrosive liquids, and more desirably magnetic responsiveness that can be droved within the water zone by magnet is placed on a high hope for the practical application of oil/water separation.In this work, superhydrophobic and superoleophilic magnetic PU sponge was fabricated by virtue of the dopamine self-polymerization to anchor Fe3O4 nanoparticles onto the skeleton of the PU sponge, followed by the introduction of the low-surface-energy hydrophobic molecules FAS-17 on the sponge surface. The sticky dopamine was chosen because it can easily adhere to all kinds of inorganic and organic surfaces and is regarded to be effective and general for immobilizing nanoparticles on solid surfaces under mild wet conditions.23,24 The involvement of magnetic particles have not only provided magnetic responsiveness, but also enhanced surface roughness. The low-surface-energy molecules FAS-17 was modified on the sponge surface to induce the superhydrophobic transformation. The magnetic PU sponge has the merits of high absorption capacity, magnetic responsiveness, anti-corrosion stability, recyclability, low cost as well as excellent superhydrophobicity and superoleophilicity. The oil/water separation experiment were carried out under magnetic actuation not only for floating oils on water surface and heavy organic pollutants under water, but also in more complex environments such as acidic solution (pH = 1), simulated seawater and basic solution (pH = 14).
2. Experimental section
2.1. Reagents and materials
PU sponge was obtained from Qirui sheet supplier (China). Fe3O4 nanoparticles and dopamine hydrochloride were purchased from Sigma-Aldrich Co. Ltd. FAS-17 [CF3(CF2)7(CH2)2Si(OCH3)3] was obtained from Gelest Inc. Morrisville, PA. Tris (hydroxymethyl) aminomethane, anhydrous ethanol, sulfuric acid, potassium dichromate and other reagents were of analytical grade and used as received without further purification. Doubly deionized water was used throughout all experiments. The oils and organic reagents were used as commercially received. The simulated seawater was configured according to the standard practice for the preparation of substitute ocean water.2.2. Preparation of magnetic PU sponge
Initially, a small piece of PU sponge (5 mm × 10 mm × 10 mm; weight ≈ 0.060 g) was immersed in CrO3/H2SO4 solution for 1 min and washed ultrasonically with double distilled water and ethanol in turn for three times to remove surface stains and oils. Then, 20 mg of Fe3O4 nanoparticles were dispersed ultrasonically in ethanol and introduced drop-by-drop in the PU sponge. After thorough drying in air, the sponge was immersed into 20 ml of dopamine solution (2 mg ml−1, ∼pH 8.5) for 24 h and later thoroughly washed with double distilled water and dried. After that, the magnetic sponge was modified with an ethanol solution of FAS-17 (1.0%) at room temperature for 24 h via a dip-coating method. For simplicity, this obtained sample is designated as “magnetic PU sponge”. For comparison, PU sponge with only FAS modification was prepared and named as “blank PU sponge”. The experimental scheme of the preparation of superhydrophobic and superoleophilic magnetic PU sponge is shown in Fig. 1.2.3. Characterization
For composition and structural investigation, X-ray diffraction (XRD) pattern of magnetic PU sponge was obtained from a Shimadzu XRD-6000 diffractometer with Cu Kα radiation (λ = 1.5406 Å). The scanning electron microscope (SEM) images were taken using a Hitachi SU8010 emission electron microscope at an accelerating voltage of 5 kV. The wetting properties of the blank and magnetic PU sponge were investigated by static contact angle measurements at ambient temperature using DM300 contact angle goniometer with a droplet volume of 5 μl. The contact angle values were measured on the three different areas of the sponge and the average value is reported here. The oil absorption ability (k) of the magnetic PU sponge was calculated through the weight measurements as per protocol as reported in the literature.253. Results and discussion
3.1. Characterization of the composition and structure
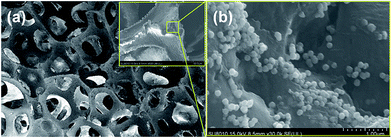
Superhydrophobic and superoleophilic magnetic PU sponge was fabricated via a two-step procedure. The first step was to anchor Fe3O4 nanoparticles onto the skeleton of the PU sponge via the self-polymerization of dopamine. The loading amount of Fe3O4 could be easily controlled by the concentration of ethanol solution of Fe3O4. The second step was to modify the low-surface-energy hydrophobic molecules FAS-17 on the sponge surface, which induced the superhydrophobic transformation. Compared with the light-yellow color of pristine PU sponge, the magnetic PU sponge became black presumably due to the involvement of Fe3O4 nanoparticles (Fig. S1†).The presence of Fe3O4 nanoparticles within the magnetic PU sponge was further investigated by XRD analysis. As Fig. 2 showed, all diffraction peaks and positions of commercially received Fe3O4 powder matched well with those from the JCPDS card (File no. 19-0629) for cubic phase magnetite. The magnetic PU sponge presented the same characteristic peaks and positions with commercial Fe3O4 powder, suggesting the successful magnetic loading. Besides, SEM images of the magnetic PU sponge with different magnifications were shown in Fig. 3. The magnetic PU sponge exhibited a highly porous and well interconnected three-dimensional network-like structure (Fig. 3a). High magnification SEM observation in Fig. 3b clearly presented that Fe3O4 nanoparticles (∼100 nm) were randomly located in the porous structure of PU sponge with partly aggregation, forming a hierarchical micro/nano-structure. Therefore, SEM and XRD analyses confirmed the Fe3O4 nanoparticles were anchored onto the PU skeleton after the dopamine self-polymerization process. The involvement of magnetic particles have enhanced surface roughness as well as provided magnetic responsiveness.
3.2. Wetting properties
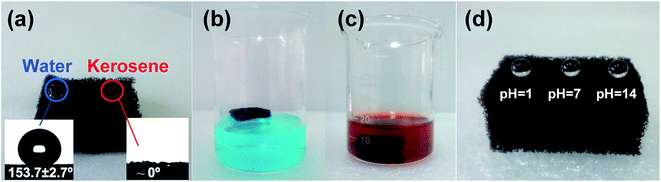
The wetting properties of magnetic PU sponge was studied by contact angle measurement. As shown in Fig. 4a, after magnetic and chemical modification with FAS-17, water droplets deposited on the surface formed almost perfect spheres and the sponge showed superhydrophobic behavior with a water contact angle of 153.7 ± 2.7° (inset of Fig. 4a). For comparison, the water contact angle of blank PU sponge was 143.0 ± 1.6°. The effect of magnetic loading amount on the water contact angle was examined and the results showed that the magnetic PU sponge retained superhydrophobicity when the mass fraction of Fe3O4 was from 3% to 32% (Table. S1†). Therefore, the superhydrophobicity of the magnetic PU sponge was presumed to benefit from the cooperation of surface chemical compositions and hierarchical structures, which ensured high efficiency in absorbing oils and organic solvents. On the other hand, oil droplets (here, kerosene) dropped on the surface of magnetic PU sponge were absorbed completely and the oil contact-angle was about 0°, showing the superoleophilic property (inset of Fig. 4a). This could be attributed to the formation of Wenzel state, i.e. fully wetting interface resulting in the absorption. Furthermore, the magnetic PU sponge remained floating on water even after 24 h, owing to its characteristics of superhydrophobicity and light weight (water stained with methylene blue for clear appearance, Fig. 4b); it could be handily moved under magnetic actuation (Movie S1†). When it was thrown into oil (oil stained with Sudan Red), it absorbed the oil and got settled down in the bottom within a short time, demonstrating its superoleophilic property (Fig. 4c). The resistance of magnetic PU sponge towards corrosive liquids was also examined. Fig. 4d showed the representative digital images of acidic (HCl solution, pH = 1), salt (NaCl solution, pH = 7), and basic (NaOH solution, pH = 14) droplets on the magnetic sponge surface. All these aqueous solution droplets presented uniformly spherical shapes and the static contact angles were almost unchanged over a wide range of pH values from 1 to 14. After it was immersed in corrosive liquids for 24 h, it seemed like more stable in acidic solution than in basic solution (Fig. S2†). The contact angles of the magnetic sponge were decreased to 130–150° depending on different corrosive liquids, but still maintained the hydrophobicity suitable for the oil/water separation. The unique wettability towards corrosive solutions resulted from the formation of air layer between the droplet and the sponge, which acted as the effective barrier to inhibit the penetration of the corrosion solution.26 Therefore, the magnetic PU sponge demonstrated excellent anti-corrosion ability, as well as simultaneously water repellence and oil absorption properties within broad magnetic loading, which is essential for the complex oily wastewater separation.3.3. Oil absorption performance
The eight different types of oils and organic solvents were tested to examine the absorption efficiency of the magnetic PU sponge. Benzene represent the most common nonpolar volatile organic compound while THF is polar volatile solvent; gasoline and kerosene are selected to represent low-viscosity oils while lubricating oil is a representative of high-viscosity oils. The oil absorbance capacity (k) of the magnetic PU sponge was determined by weight-gain ratio according to the following formula.27 The dry magnetic PU sponge was cut into the same size and initially weighted as W0; after that, it was immersed into different oils and organic solvents. The soaked PU sponges were removed from the oils at certain internals and their weights were monitored as a function of time (Wt).| k = (Wt − W0)/W0 |
The absorption kinetic curves of the magnetic PU sponge were shown in Fig. S3.† The absorption rate of the magnetic PU sponge towards gasoline, n-hexane, THF and benzene is so fast and could reach absorption equilibrium within 5 seconds. As for the high-viscosity lubricating oil, it requires at least 5 min to reach equilibrium because the absorption process is dominated by the diffusion step.28 The magnetic PU sponges were immersed into different oils and organic solvents for 5 min and the saturated absorption capacity was shown in Fig. 5. It is observed that the magnetic PU sponge could absorb different oils and organic solvents at 10 to 35 times of its own weight due to different viscosity and density of oils and organic solvents.29 The high efficiency of magnetic PU sponge was regarded to benefit from the simultaneous excellent superhydrophobicity and superoleophilicity as well as its abundant 3D porous architecture.
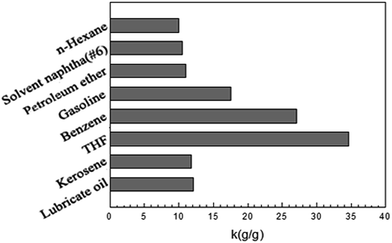 | ||
| Fig. 5 The saturated absorption capacity of the magnetic PU sponge into various oils and organic solvents. | ||
3.4. Oil/water separation
The oil/water separation experiment of magnetic PU sponge was performed as follows. As shown in Fig. S4,† manipulated by a magnet bar, magnetic PU sponge approached the oil/water mixture (lubricating oil dyed with Sudan Red), and selectively and rapidly absorbs floating oil on water surface, only leaving water behind. In addition, it could be also used for the removal of heavy organic pollutants under water. For example, when chloroform (dyed with Sudan Red) mixed with water, chloroform was located in the bottom due to the higher density (Fig. 6a). The magnetic PU sponge still tended to float on the surface of water. Once immersed in water by an external force, the sponge exhibited a silver mirror-like surface because of being surrounded by air cushion by repelling the water (Fig. 6b), and selectively absorbed immiscible chloroform in the bottom from the mixture (Fig. 6c–e). More favorably, chloroform could be recovered via a simple mechanical squeezing procedure by taking the advantage of the excellent elastic property of the sponge and the sponge could be reused repeatedly (Fig. 6f–h). Furthermore, no trace of water was visible in the collected chloroform by the naked eye, exhibiting high separation efficiency. Judging from this, it is not difficult to speculate that the magnetic PU sponge could also efficiently and conveniently clean up heavy oils under water as well as floating oils on water surface.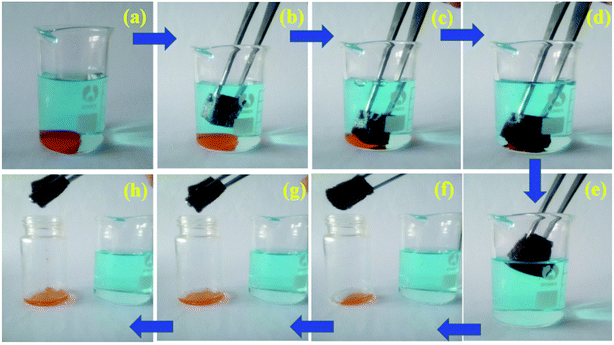 | ||
| Fig. 6 Optical images demonstrating organic solvent (e.g. chloroform dyed with Sudan Red) collection process from water using magnetic PU sponge. | ||
To further investigate the oil/water separation performance in the more complex environment, three kinds of the solutions such as acidic solution (pH = 1), simulated seawater and basic solution (pH = 14) were prepared, respectively. Compared with the standard saturated absorption capacity of the magnetic PU sponge towards lubricating oil (k0), the absorption capacity of the sponge towards lubricating oil in the simulated seawater was nearly close to k0, similar to the acidic solution and better than in the basic solution (Fig. 7a). This might because the basic solution had a big effect on the hydrophobicity of the sponge. Moreover, Fig. 7b showed that the magnetic sponge exhibited excellent separation performance under magnetic actuation, suggesting the great potential in the practical oily wastewater treatment.
3.5. Reusability
In practical applications, recyclability is considered to be another important parameter for absorbent materials. The reusability of magnetic PU sponge was investigated by repeated testing of absorption capacity in benzene medium. The weight of magnetic PU sponge was measured before and after every cycle. Fig. 8 shows the weight of the sponge every cycle and its absorbance capacity towards benzene remained almost unchanged even after 10 times of cycling, suggesting the excellent recyclability of magnetic PU sponge. On the other hand, to illustrate the role of the dopamine, the magnetic PU sponge but without dopamine treatment was measured in the same condition. Results showed that the absorption capacity of the sponge decreased to 30% after 4 times of cycling because of the gradual loss of magnetic nanoparticles as evidenced by SEM images (Fig. S5†). This proved the implementation of dopamine self-polymerization was necessary and efficient for the immobilization of Fe3O4 nanoparticles.4. Conclusions
In summary, the magnetic PU sponge was successfully fabricated via magnetic anchoring with the help of the dopamine self-polymerization and hydrophobic covalently grafting with FAS-17. It displays high water repellency and superior stability against various corrosive solutions over a wide range of pH values. More favorably, the magnetic PU sponge exhibits attractive magnetic responsiveness and gave the outstanding separation performance with high absorption capacity (up to 35 times of its own weight) under magnetic actuation, not only for floating oils on water surface and heavy oils under water, but also in more complex environment such as acidic solution (pH = 1) and simulated seawater. The absorbed oils in the sponge could be collected by simple mechanical squeezing because of the great elasticity of the sponge. The raw materials such as PU sponge, Fe3O4 powder could be commercially available on a large scale and this approach is more convenient and economical without harsh conditions, sophisticated equipment, or aggressive etching solution involved, which will dramatically advance practicality for mass production and thus their applications in cleanup of oil spills and removal of organic solvents from water.Acknowledgements
We greatly appreciate the support of the National Natural Science Foundation of China (21101056, 21105021).References
- C. Ruan, K. Ai, X. Li and L. Lu, Angew. Chem., Int. Ed., 2014, 53, 5556–5560 CrossRef CAS PubMed.
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Marinas and A. M. Mayes, Nature, 2008, 452, 301–310 CrossRef CAS PubMed.
- A. B. Nordvik, J. L. Simmons, K. R. Bitting, A. Lewis and T. Strøm-Kristiansen, Spill Sci. Technol. Bull., 1996, 3, 107–122 CrossRef CAS.
- L. Zhang, Z. Zhang and P. Wang, NPG Asia Mater., 2012, 4, e8 CrossRef PubMed.
- Q. Zhu and Q. Pan, ACS Nano, 2014, 8, 1402–1409 CrossRef CAS PubMed.
- I. Buist, S. Potter, T. Nedwed and J. Mullin, Cold Reg. Sci. Technol., 2011, 67, 3–23 CrossRef PubMed.
- S. R. Jadhav, P. K. Vemula, R. Kumar, S. R. Raghavan and G. John, Angew. Chem., Int. Ed., 2010, 49, 7695–7698 CrossRef CAS PubMed.
- E. B. Kujawinski, M. C. Kido Soule, D. L. Valentine, A. K. Boysen, K. Longnecker and M. C. Redmond, Environ. Sci. Technol., 2011, 45, 1298–1306 CrossRef CAS PubMed.
- H.-C. Yang, J.-K. Pi, K.-J. Liao, H. Huang, Q.-Y. Wu, X.-J. Huang and Z.-K. Xu, ACS Appl. Mater. Interfaces, 2014, 6, 12566–12572 CAS.
- L. Feng, Z. Zhang, Z. Mai, Y. Ma, B. Liu, L. Jiang and D. Zhu, Angew. Chem., 2004, 116, 2046–2048 CrossRef PubMed.
- Y. Dong, J. Li, L. Shi, X. Wang, Z. Guo and W. Liu, Chem. Commun., 2014, 50, 5586–5589 RSC.
- M. O. Adebajo, R. L. Frost, J. T. Kloprogge, O. Carmody and S. Kokot, J. Porous Mater., 2003, 10, 159–170 CrossRef CAS.
- Y. Chu and Q. Pan, ACS Appl. Mater. Interfaces, 2012, 4, 2420–2425 CAS.
- F. Wang, S. Lei, M. Xue, J. Ou, C. Li and W. Li, J. Phys. Chem. C, 2014, 118, 6344–6351 CAS.
- D. D. Nguyen, N.-H. Tai, S.-B. Lee and W.-S. Kuo, Energy Environ. Sci., 2012, 5, 7908–7912 CAS.
- C.-F. Wang and S.-J. Lin, ACS Appl. Mater. Interfaces, 2013, 5, 8861–8864 CAS.
- X. Gui, J. Wei, K. Wang, A. Cao, H. Zhu, Y. Jia, Q. Shu and D. Wu, Adv. Mater., 2010, 22, 617–621 CrossRef CAS PubMed.
- J. Li, L. Yan, H. Li, J. Li, F. Zha and Z. Lei, RSC Adv., 2015, 5, 53802–53808 RSC.
- X. Zhang, Z. Li, K. Liu and L. Jiang, Adv. Funct. Mater., 2013, 23, 2881–2886 CrossRef CAS PubMed.
- H. Wang, E. Wang, Z. Liu, D. Gao, R. Yuan, L. Sun and Y. Zhu, J. Mater. Chem. A, 2015, 3, 266–273 CAS.
- N. Chen and Q. Pan, ACS Nano, 2013, 7, 6875–6883 CrossRef CAS PubMed.
- P. Calcagnile, D. Fragouli, I. S. Bayer, G. C. Anyfantis, L. Martiradonna, P. D. Cozzoli, R. Cingolani and A. Athanassiou, ACS Nano, 2012, 6, 5413–5419 CrossRef CAS PubMed.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426–430 CrossRef CAS PubMed.
- Y. Cao, Y. Chen, N. Liu, X. Lin, L. Feng and Y. Wei, J. Mater. Chem. A, 2014, 2, 20439–20443 CAS.
- Q. Zhu, Q. Pan and F. Liu, J. Phys. Chem. C, 2011, 115, 17464–17470 CAS.
- X. Jin, B. Shi, L. Zheng, X. Pei, X. Zhang, Z. Sun, Y. Du, J. H. Kim, X. Wang, S. Dou, K. Liu and L. Jiang, Adv. Funct. Mater., 2014, 24, 2721–2726 CrossRef CAS PubMed.
- Q. Zhu, F. Tao and Q. Pan, ACS Appl. Mater. Interfaces, 2010, 2, 3141–3146 CAS.
- Y. Pan, K. Shi, C. Peng, W. Wang, Z. Liu and X. Ji, ACS Appl. Mater. Interfaces, 2014, 6, 8651–8659 CAS.
- Y. Liu, J. Ma, T. Wu, X. Wang, G. Huang, Y. Liu, H. Qiu, Y. Li, W. Wang and J. Gao, ACS Appl. Mater. Interfaces, 2013, 5, 10018–10026 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra12301a |
| This journal is © The Royal Society of Chemistry 2015 |