Preparation of UV-curable functionalized phosphazene-containing nanotube/polyurethane acrylate nanocomposite coatings with enhanced thermal and mechanical properties†
Shuilai Qiua,
Siyu Lib,
Youji Taoc,
Xiaming Fengad,
Bin Yuad,
Xiaowei Mua,
Weiyi Xing*a,
Yuan Hu*ad and
Ganxin Jiec
aState Key Laboratory of Fire Science, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, P. R. China. E-mail: yuanhu@ustc.edu.cn; xingwy@ustc.edu.cn; Fax: +86-551-63601664, +86-551-63602353; Tel: +86-551-63601664, +86-551-63602353
bDepartment of Polymer Science and Engineering, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, P. R. China
cState Key Laboratory of Environmental Adaptability for Industrial Products, China National Electric Apparatus Research Institute, Guangzhou, Guangdong 510300, P. R. China
dSuzhou Key Laboratory of Urban Public Safety, Suzhou Institute for Advanced Study, University of Science and Technology of China, 166 Ren'ai Road, Suzhou, Jiangsu 215123, P. R. China
First published on 25th August 2015
Abstract
Poly(cyclotriphosphazene-co-4,4′-sulfonyldiphenol) (PZS) nanotubes with active hydroxyl groups were fabricated via an in situ template method under mild conditions, and then modified by acryloyl chloride to obtain the acryloyl-group functionalized PZS (f-PZS) nanotubes. The structure of the PZS nanotubes was characterized by Fourier transform infrared spectroscopy and the morphology was investigated by scanning electron microscopy and transmission electron microscopy. The f-PZS/polyurethane acrylate (f-PZS/PUA) nanocomposite coatings were prepared by UV radiation technology to covalently introduce f-PZS nanotubes into a PUA matrix. Dynamic mechanical analysis and tensile tests were performed to characterize the mechanical properties of the f-PZS/PUA nanocomposite coatings. The optimal reinforcing effect for the PUA matrix was observed when the content of f-PZS nanotubes was 3.0 wt%. The thermal stability of the PUA nanocomposites was studied by thermo gravimetric analysis. It indicates that the onset thermal degradation temperature of the f-PZS/PUA nanocomposites with 1.0 wt% f-PZS nanotubes is increased by 36.3 °C. These remarkable property reinforcements are attributed to the covalent functionalization of PZS nanotubes, which can effectively improve the interfacial interaction between the f-PZS nanotubes and the PUA matrix.
1. Introduction
Over the past few decades, UV-curing technology has been commonly used in various industrial sectors to achieve ultrafast hardening of protective coatings, printing inks, adhesives, varnishes and composites,1–3 because of its distinctive advantages over thermal curing, such as low energy consumption, easy operation, environmental friendliness (low/zero emissions) and efficiency (seconds or minutes).4–6 Among various UV-curable resins, polyurethane acrylate (PUA) has attracted great interest in coatings and adhesives due to its unique properties, such as outstanding adhesion, excellent flexibility and chemical resistance.7–9 However, UV-curable PUA coatings show poor thermal stability and mechanical strength in practical applications. It is thereby essential to fabricate modified PUA composites with enhanced thermal and mechanical properties to broaden their application. Therefore, the prior works have been focused on enhancing these properties of PUA by incorporating various kinds of nanofillers into matrices, such as carbon nanotubes, nano-silica, nano-clay and graphene,10–14 but these nanofillers always aggregate together due to their poor dispersibility in organic matrices, which badly restrains the effect of improvements. On the base of these works, developing a new kind of nanofillers without complex functionalization in mild conditions, which can be well dispersed in polymer matrices is of great importance in the practical applications.Phosphazene-containing polymers are versatile hybrid organic–inorganic materials that have many outstanding properties. Presence of the inorganic phosphazene (–P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) unit in macromolecular backbones and the structural multiplicity of side groups, such as organic, organometallic or inorganic units, provides tremendous flexibility to functionalize the materials through physical or chemical modifications for numerous applications.15–20 It is well known that phosphazene-containing polymers have been widely used as the optical materials, biomaterials, membrane materials, electrical and electrochromic materials, hybrid materials, etc., because of their superior high thermal stability and biocompatibility of the inorganic unit and inconceivable structural diversity.21–24 In the previous study, the fabrication of micro- and nanoscale cyclotriphosphazene-containing polymers was reported, such as poly(cyclotriphosphazene-co-4,4′-sulfonyldiphenol) (PZS) nanotubes, microspheres, nanochains and nanofibers.25–29 These synthesized nanomaterials possess high thermal stability, radiation resistance and fire retardancy.30–32 Owing to the organic–inorganic hybrid structure, it is easy for PZS nanotubes to be well dispersed in polymer matrices. By changing the reaction condition and molar ratio of monomers, the PZS nanotubes with active hydroxyl groups were synthesized via an in situ template approach.33,34 Due to the hydroxyl groups of PZS nanotubes providing a platform for surface functionalization with other active molecules or molecular chains, it is convenient to modify the PZS nanotubes by introducing specific functional groups via covalent or noncovalent methods to acquire several expectant achievements.35 For example, the introduction of PZS nanotubes into PUA is expected to have a homogeneous dispersion in matrices and create a CNT-like reinforcement effect.36 Up to now, there is no report about the covalent incorporation of functionalized PZS (f-PZS) nanotubes into PUA matrices by UV radiation technology.
N–) unit in macromolecular backbones and the structural multiplicity of side groups, such as organic, organometallic or inorganic units, provides tremendous flexibility to functionalize the materials through physical or chemical modifications for numerous applications.15–20 It is well known that phosphazene-containing polymers have been widely used as the optical materials, biomaterials, membrane materials, electrical and electrochromic materials, hybrid materials, etc., because of their superior high thermal stability and biocompatibility of the inorganic unit and inconceivable structural diversity.21–24 In the previous study, the fabrication of micro- and nanoscale cyclotriphosphazene-containing polymers was reported, such as poly(cyclotriphosphazene-co-4,4′-sulfonyldiphenol) (PZS) nanotubes, microspheres, nanochains and nanofibers.25–29 These synthesized nanomaterials possess high thermal stability, radiation resistance and fire retardancy.30–32 Owing to the organic–inorganic hybrid structure, it is easy for PZS nanotubes to be well dispersed in polymer matrices. By changing the reaction condition and molar ratio of monomers, the PZS nanotubes with active hydroxyl groups were synthesized via an in situ template approach.33,34 Due to the hydroxyl groups of PZS nanotubes providing a platform for surface functionalization with other active molecules or molecular chains, it is convenient to modify the PZS nanotubes by introducing specific functional groups via covalent or noncovalent methods to acquire several expectant achievements.35 For example, the introduction of PZS nanotubes into PUA is expected to have a homogeneous dispersion in matrices and create a CNT-like reinforcement effect.36 Up to now, there is no report about the covalent incorporation of functionalized PZS (f-PZS) nanotubes into PUA matrices by UV radiation technology.
Herein, the UV-curable f-PZS/PUA nanocomposite coatings were fabricated for the first time. To improve the interface adhesion between PZS nanotubes and polymer matrices, the PZS nanotubes with active hydroxyl groups were synthesized, and were modified with acryloyl chloride in a mild condition to obtain the acryloyl-group functionalized PZS nanotubes. Subsequently, the f-PZS nanotubes were covalently introduced into PUA matrix by UV radiation technology. The reinforcement effect of f-PZS nanotubes on the mechanical and thermal properties of PUA was investigated.
2. Experimental section
2.1 Materials
Hexachlorocyclotriphosphazene (HCCP) was purchased from Aldrich (U.S.) and purified through sublimation before use. Tetrahydrofuran (THF), ethanol, acetone and triethylamine (TEA) were obtained from Sinopharm Chemical Reagent Co., Ltd (China) and dried before use. Acryloyl chloride and 4,4′-sulfonyldiphenol (BPS) were purchased from Shanghai Chemical Reagents Corp. (Shanghai, China) and used without further purification. PUA resin, an aliphatic polyurethane acrylate was purchased from DSM-AGI Co., Ltd (Taiwan, China). 2-Hydroxy-2-methyl-1-phenyl-1-propanone (Darocur 1173) was purchased from Shanghai Chemical Industry Co., Ltd (Shanghai, China), used as a photoinitiator.2.2 Synthesis of PZS nanotubes with active hydroxyl groups
In a typical synthesis procedure (Fig. 1), a given amount of BPS and TEA (2.08 g, 20.6 mmol) were dissolved in 125 mL THF under ultrasonication (53 kHz) at 40 °C in a three-necked flask equipped with a mechanical stirrer, constant pressure dropping funnel and reflux condenser. 125 mL of THF with HCCP (1.2 g, 3.45 mmol) was added dropwise to the flask within 2 h. The system was accurately controlled at 40 °C with ultrasonication for 12 h. Then the solvent was removed by distillation under reduced pressure and then the precipitate was washed with ethanol and deionized water three times, respectively. Finally, the resulting products were dried under vacuum at 80 °C, weighted and analyzed by FTIR. The yield was about 70%, calculated from HCCP. | ||
| Fig. 1 Schematic illustration of the synthetic routes of the f-PZS nanotubes and f-PZS/PUA nanocomposites. | ||
2.3 Synthesis of acryloyl-group functionalized PZS nanotubes (f-PZS)
2 g of PZS powder and 6.7 g TEA were added to 100 mL THF in a 250 mL three-necked flask. The mixture was kept in the ultrasonic bath (53 kHz) for 30 min. Then 6 g of acryloyl chloride was added dropwise through a constant pressure funnel to the flask within 2 h, and cooled with an ice-water bath. Then the temperature of water bath was raised up to 35 °C and maintained for another 12 h. The solid products were filtered, washed with ethanol and deionized water, and then dried under vacuum at 60 °C for 24 h. The schematic of synthetic procedure of the f-PZS nanotubes is shown in Fig. 1.2.4 Preparation of f-PZS/PUA nanocomposites
A typical procedure to prepare f-PZS/PUA nanocomposites with 0.1 wt% f-PZS was described as follows: f-PZS powder (10 mg) was dispersed in acetone (10 mL) in a 50 mL three-necked flask with the assistance of ultrasonic bath (53 kHz) for 0.5 h at room temperature. PUA (9.99 g) was subsequently incorporated into the above f-PZS suspension and the ultrasonic bath lasted for 2 h. The solvent was removed in a vacuum oven at 60 °C for 2 h. After cooling to room temperature, the photoinitiator Darocur 1173 (4 wt%) was uniformly added into the mixture of f-PZS/PUA blend by a vigorous stirring. Then the blend was coated on a glass plate and then dried in an oven at 60 °C for 2 h to remove the solvent completely. The film was cured by UV irradiation equipment (80 W cm−2, Lantian Co., China). Finally, the f-PZS/PUA nanocomposite coatings named as f-PZS/PUA-x were obtained according to the weight percentage of f-PZS nanotubes.2.5 Characterization
Fourier transform infrared (FTIR) spectra of PZS and f-PZS were obtained with a Nicolet 6700 spectrometer (Nicolet Instrument Co., USA). The samples were mixed with KBr powders and pressed into tablets before characterization.X-Ray photoelectron spectroscopy (XPS) test was performed with a VG ESCALAB MK-II electron spectrometer (V.G. Scientific Ltd, UK) to investigate the composition of the samples. The excitation source was an Al Kα ray at 1486.6 eV.
X-Ray diffraction (XRD) patterns were recorded on an X-ray diffractometer (Rigaku Co., Japan), using Cu Kα radiation (λ = 0.15418 nm), at a scanning rate of 4° min−1.
Thermogravimetric analysis (TGA) was conducted on a Q5000 thermo-analyzer instrument (TA Instruments Inc., USA) from 20 to 800 °C at a linear heating rate of 20 °C min−1 under nitrogen atmospheres.
The morphology of PZS and f-PZS nanotubes was examined by a JEM-2100F transmission electron microscopy (Japan Electron Optics Laboratory Co., Ltd, Japan). PZS and f-PZS nanotubes were dispersed in ethanol with ultrasonication for 0.5 h, and then dripped onto copper grids for observation.
The morphology of various PUA nanocomposites was studied by scanning electron microscopy (SEM, AMRAY 1000B, Beijing R&D center of the Chinese Academy of Sciences, China). The fractured surface was previously coated with a conductive layer of gold.
SEM observations of PZS and f-PZS nanotubes were conducted by high-resolution JEOL JSM-6700 field-emission scanning electron microscopy (FE-SEM). The samples were dispersed in ethanol with ultrasonication for 0.5 h, and then dripped onto copper sheets for observation. The studied surfaces were first sputter-coated with a thin layer of gold before the observation.
The tensile strength of PUA nanocomposites was measured on an MTS CMT6104 universal testing machine (MTS Systems Co. Ltd, P. R. China) according to the Chinese standard of GB 13022-91. The stretching rate was 100 mm min−1. Each specimen was repeated for five times.
Dynamic mechanical analysis (DMA) was performed with the PerkinElmer Pyris Diamond DMA from −80 to 150 °C at a heating rate of 5 °C min−1, at a frequency of 10 Hz in the tensile configuration.
The transparency of five samples was studied using the DUV-3700 UV-Vis spectrometer (Shimadzu, Japan). The transmission mode was used and the wavelength ranges were set from 400 to 700 nm.
3. Results and discussion
3.1 Characterization of PZS and f-PZS nanotubes
The polymerization generates PZS nanotubes in a solution of THF under ultrasonication, with TEA as the acid acceptor. The polycondensation between the HCCP and BPS produces the hydrogen chloride (HCl), which can absorbs by the TEA and formed as rodlike crystals, TEA hydrochloride (TEACl) and then accelerated the reaction. Herein, these rodlike crystals of TEACl acting as hosted core–shell structures for the crosslinking polymerization on their surfaces. When the polycondensation is completed then the TEACl core is dissolved and the nanotubes are obtained.28The FTIR spectra of PZS and f-PZS nanotubes are shown in Fig. 2. As can be seen from the spectrum of the bare PZS nanotubes, two sharp peaks at 1590 and 1488 cm−1 are assigned to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C group in the phenylene of sulfonyldiphenol units. The strong peaks at 1186 and 883 cm−1 are associated with the P
C group in the phenylene of sulfonyldiphenol units. The strong peaks at 1186 and 883 cm−1 are associated with the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and P–N characteristic absorption of cyclotriphosphazene, respectively. The characteristic absorption of the O
N and P–N characteristic absorption of cyclotriphosphazene, respectively. The characteristic absorption of the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in sulfonyldiphenol units can be observed at 1293 and 1153 cm−1. The absorption peak at 941 cm−1 corresponds to the P–O–Ar band, which provides obvious evidence for the occurrence of polycondensation reaction between the comonomers HCCP and BPS. Furthermore, the peaks at 3100 and 3073 cm−1 are attributed to the stretching vibration of the hydroxyl in phenolic group. Obviously, after the modification of PZS nanotubes with acryloyl groups, the intensive peak at 1726 cm−1 corresponding to C
O group in sulfonyldiphenol units can be observed at 1293 and 1153 cm−1. The absorption peak at 941 cm−1 corresponds to the P–O–Ar band, which provides obvious evidence for the occurrence of polycondensation reaction between the comonomers HCCP and BPS. Furthermore, the peaks at 3100 and 3073 cm−1 are attributed to the stretching vibration of the hydroxyl in phenolic group. Obviously, after the modification of PZS nanotubes with acryloyl groups, the intensive peak at 1726 cm−1 corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and the peak at 1631 cm−1 corresponding to C
O and the peak at 1631 cm−1 corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in the acryloyl groups are observed in Fig. 2. These results demonstrate that acryloyl chloride has reacted with the active –OH in PZS nanotubes and the nanotubes have been successfully modified.
C in the acryloyl groups are observed in Fig. 2. These results demonstrate that acryloyl chloride has reacted with the active –OH in PZS nanotubes and the nanotubes have been successfully modified.
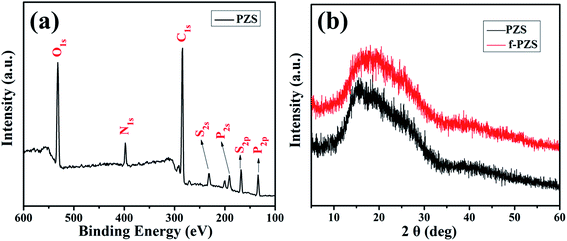
XPS analysis was carried out to evaluate the elemental composition of the as-synthesized products. Fig. 3a shows the XPS wide scan spectrum of PZS nanotubes. Obviously, the elements of nanotubes surface was composited of C, O, P, N, and S. Meanwhile, the atom concentration of N, P, and S was 5.55%, 5.76%, and 4.87%, respectively. The mole ratio of N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P
P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S was similar with the theoretical value for PZS with highly cross-linked chemical structure, indicating the successful preparation of PZS nanotubes.37 XRD pattern for PZS and f-PZS nanotubes further confirmed the above results. As shown in Fig. 3b, the very broad diffraction peak at 2θ values around 15.0° corresponds to the reflection peak position of pure PZS nanotubes, consistent with previous work.31 For f-PZS nanotubes, the position and intensity of diffraction peak slightly changed, it reveals that f-PZS nanotubes maintain the same crosslinking structure of pure PZS.
S was similar with the theoretical value for PZS with highly cross-linked chemical structure, indicating the successful preparation of PZS nanotubes.37 XRD pattern for PZS and f-PZS nanotubes further confirmed the above results. As shown in Fig. 3b, the very broad diffraction peak at 2θ values around 15.0° corresponds to the reflection peak position of pure PZS nanotubes, consistent with previous work.31 For f-PZS nanotubes, the position and intensity of diffraction peak slightly changed, it reveals that f-PZS nanotubes maintain the same crosslinking structure of pure PZS.
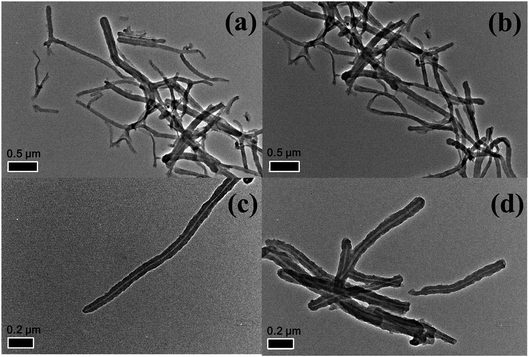
FE-SEM was employed to investigate the morphology and microstructure of PZS (Fig. 4a and c) and f-PZS nanotubes (Fig. 4b and d). As shown in Fig. 4a and c, the nanotubes exhibit a crosslinking network structure with noodle-like fiber shape entangled with each other, which are similar to the previous report.37 Typically, the PZS nanotubes are several micrometers in length. Most of them have an outer diameter of about 40–50 nm. After modified with acryloyl groups (Fig. 4b and d), there is not obvious change observed for outer diameter and surface character of the f-PZS nanotubes.
The TEM images of PZS and f-PZS nanotubes are shown in Fig. 5. The pure PZS nanotubes are showed as like noodles (Fig. 5a and c) and which are possess the hollow tubular structures. Majority of the tubes are several micrometers in length and their outer diameters are about 40–50 nm, and most of the tube ends are closed. After modified with acryloyl groups, the outer diameters of the f-PZS nanotubes (Fig. 5b and d) seem not being changed a lot but the surface is obviously rougher than that of bare PZS nanotubes. It is mainly because the small molecular acryloyl groups interspersed on the PZS nanotubes cannot cover the whole nanotube, and form a new layer coated on the PZS nanotubes. When the f-PZS nanotubes were added to PUA matrix, the acryloyl groups interspersing on the nanotubes, which can addition reacted with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in PUA to make the nanotubes firmly exist in the matrix to reinforce the material.
C bonds in PUA to make the nanotubes firmly exist in the matrix to reinforce the material.
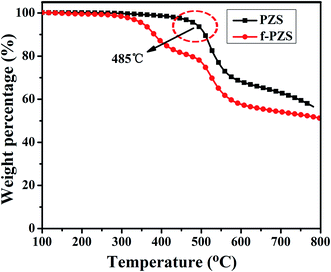
Thermal stability of PZS and f-PZS nanotubes was investigated by TGA under nitrogen atmosphere. As can be observed in Fig. 6, the initial mass loss of bare PZS nanotubes over 485 °C reveals that the PZS nanotubes exhibit superior thermal stability, which is benefited from their covalently cross-linked network structure. For f-PZS nanotubes, the mass loss in the region of 350–480 °C, is attributed to the decomposition of labile oxygen functional groups at earlier stage. However, the initial decomposition of f-PZS nanotubes as substrate starts at approximately 480 °C, is slightly lower than that of bare PZS nanotubes, which reveals the surface modification has a slight effect on the initial decomposition temperature of PZS nanotubes. After thermal degradation of f-PZS nanotubes in nitrogen, the final char residue at 800 °C is about 52 wt%, which is little lower than that of pure PZS nanotubes, a char yield of 57 wt%. The results indicate that the successful organic modification of PZS nanotubes and the outstanding thermal stability of f-PZS nanotubes, which is derived from their special molecular hybrid network structures and the inherent thermal stability of the cyclotriphosphazene.
3.2 Fractured surface morphology of PUA and f-PZS/PUA nanocomposites
Fig. 7 shows the SEM images of the cross-section of pure PUA (a) and f-PZS/PUA-3.0 (b) nanocomposites. It can be clearly seen that the fractured surface of neat PUA is quite smooth, while that of f-PZS/PUA-3.0 nanocomposite sample is very rough. The f-PZS nanotubes were covalently introduced into PUA matrix by UV radiation technology, which can result in the strong interfacial interaction between two materials. It is also worthy to note that a uniform dispersion of the PZS nanotubes within polymer matrix is achieved. This phenomenon is in good agreement with the previous literature.38 | ||
| Fig. 7 SEM micrographs of the fractured sections of: neat PUA (a) and f-PZS/PUA-3.0 nanocomposite (b). | ||
3.3 Thermal properties of PUA and f-PZS/PUA nanocomposites
The TGA curves of PUA and f-PZS/PUA nanocomposites under nitrogen gas condition are shown in Fig. 8. The onset degradation temperature (Td) defined as the temperature at 5 wt% mass loss. The thermal degradation process of f-PZS/PUA nanocomposites in nitrogen atmosphere mainly shows a single mass-loss stage in ranges of 300 to 460 °C, which is attributed to the degradation of PUA matrices. Compared to pure PUA, the f-PZS/PUA composites exhibit higher thermal stability. As the content of the f-PZS nanotubes increases from 0.1 to 3.0 wt%, the Td is increased by 7.2–36.3 °C, the maximum increase of Td is approximately 36.3 °C (f-PZS/PUA-1.0). The improvement of thermal stability can be attributed by two key factors: crosslinking structure from the f-PZS nanotubes introducing into PUA matrices; and the random distributed PZS nanotubes connected with each other to form network structure. In the decomposition process, the network structure acts as a physical barrier to effectively cut off the heat and mass transfer between the inner materials and the surroundings, thereby delaying the thermal degradation of polymer matrices. Meanwhile, the char residual of f-PZS/PUA nanocomposites at 800 °C slightly increase as the content of f-PZS nanotubes increasing.3.4 Mechanical properties of PUA and f-PZS/PUA nanocomposites
Fig. 9 shows the representative stress–strain curves for pure PUA and f-PZS/PUA nanocomposites. As can be seen from Fig. 9, the ever-increasing tensile strength of nanocomposite films is originated from the increased f-PZS nanotubes content. Compared to the tensile strength of pure PUA (1.1 MPa), it has a slight increase by 0.8 MPa for that of f-PZS/PUA-0.1 nanocomposite film. It achieves to 3.7 MPa as the highest point when the content increases to 3.0 wt%. Meanwhile, the changes of elongation at break display a similar trend to that of tensile strength for the f-PZS/PUA nanocomposite films. The increase in elongation at break is observed for all of f-PZS/PUA nanocomposite samples relative to pristine PUA film. This reinforcement effect can be explained by the CNT-like effect, the PZS nanotubes can be introduced to PUA to reinforce matrix as they are expected to have a homogeneous dispersion, especially due to the strong interfacial interaction between the f-PZS nanotubes and PUA matrix resulted from the chemical bonding reaction, and this chemical bonding enables the load transfer from the ductile polymer to the rigid inorganic phase to reduce the slippage during straining. Furthermore, this interaction may be able to change the static (or strained) microphase morphology of PUA matrix in such a way that results in enhanced mechanical properties.39As is well known, PZS nanotubes are considered as the effective reinforcing nanofillers for polymer composites. Therefore, DMA was carried out to investigate the influence of f-PZS nanotubes on the dynamic mechanical properties of f-PZS/PUA nanocomposites. Fig. 10 and 11 provide the DMA traces: temperature dependence of storage modulus (E′) and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ for the PUA and f-PZS/PUA nanocomposites. The temperature at the peak of loss factor tan
δ for the PUA and f-PZS/PUA nanocomposites. The temperature at the peak of loss factor tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ curve is defined as the glass transition temperature (Tg).40 As shown in Fig. 10, the incorporation of f-PZS nanotubes results in an increase of storage modulus throughout the relative low temperature ranges. Compared to pure PUA, the E′ at −75 °C for f-PZS/PUA-0.1, f-PZS/PUA-0.5, f-PZS/PUA-1.0 and f-PZS/PUA-3.0 is increased by 10.8%, 26.6%, 37.9% and 45.0%, respectively, which can be attributed to the high stiffness of PZS nanotubes and the interfacial interactions between the f-PZS nanotubes and PUA matrices. It can be seen obviously that the Tg values of the cured films decrease with increasing content of f-PZS nanotubes (Fig. 11). When the f-PZS nanotubes content increases from 0.1 wt% to 3 wt%, the Tg value of PUA nanocomposite film decreases from 13 °C to 0.5 °C. This is probably due to Tg is related to free volume of the materials, incorporation of f-PZS nanotubes can increase the free volume in PUA composites, and the composites with higher additive amount of nanofillers has a higher free volume fraction, which can result in lower Tg value.41 In summary, in comparison with pure PUA film, the incorporation of f-PZS nanotubes is effective in reinforcing the mechanical properties of f-PZS/PUA nanocomposites by increasing the tensile strength and E′.
δ curve is defined as the glass transition temperature (Tg).40 As shown in Fig. 10, the incorporation of f-PZS nanotubes results in an increase of storage modulus throughout the relative low temperature ranges. Compared to pure PUA, the E′ at −75 °C for f-PZS/PUA-0.1, f-PZS/PUA-0.5, f-PZS/PUA-1.0 and f-PZS/PUA-3.0 is increased by 10.8%, 26.6%, 37.9% and 45.0%, respectively, which can be attributed to the high stiffness of PZS nanotubes and the interfacial interactions between the f-PZS nanotubes and PUA matrices. It can be seen obviously that the Tg values of the cured films decrease with increasing content of f-PZS nanotubes (Fig. 11). When the f-PZS nanotubes content increases from 0.1 wt% to 3 wt%, the Tg value of PUA nanocomposite film decreases from 13 °C to 0.5 °C. This is probably due to Tg is related to free volume of the materials, incorporation of f-PZS nanotubes can increase the free volume in PUA composites, and the composites with higher additive amount of nanofillers has a higher free volume fraction, which can result in lower Tg value.41 In summary, in comparison with pure PUA film, the incorporation of f-PZS nanotubes is effective in reinforcing the mechanical properties of f-PZS/PUA nanocomposites by increasing the tensile strength and E′.
 | ||
| Fig. 10 Storage modulus (E′) curves of the PUA and f-PZS/PUA nanocomposites as a function of temperature. | ||
3.5 UV-Vis analysis of UV-cured f-PZS/PUA nanocomposite films
The UV-Vis spectra of PUA and f-PZS/PUA nanocomposites are shown in Fig. 12, which is inserted with the digital photos of PUA nanocomposite films with different f-PZS contents. It can be observed that pure PUA film shows high transparency throughout the visible light range of 400–700 nm, while the transmittance values of f-PZS/PUA nanocomposite films are decreased gradually along with the increasing of f-PZS content. The transmittance value at 550 nm is about 90.2% and 79.4% for the f-PZS/PUA-0.1 and f-PZS/PUA-3.0 samples, respectively, whereas that of pure PUA film is 91.5%. Relative reduction of the transmittance is probably attributed to the shielding effect of PZS nanotubes in PUA matrices. The high transmittance is an assessment for the formation of uniform phase. It reveals that the UV-cured f-PZS/PUA nanocomposite films present considerable optical transparency and compatibility, which are important characteristics for their application as the protective coatings.4. Conclusions
Novel acryloyl-group modified phosphazene-containing nanotubes (f-PZS) were successfully fabricated and were used to reinforce PUA matrix. Owing to the homogeneous dispersion of f-PZS nanotubes in PUA matrix and the strong interfacial interactions between f-PZS nanotubes and polymer matrix, the f-PZS/PUA nanocomposites exhibit dramatically enhancement on the thermal stability and mechanical properties. The DMA result shows that the storage modulus of f-PZS/PUA-3.0 nanocomposite at −75 °C is increased by 45.0%, compared to that of pure PUA. The addition of f-PZS nanotubes into PUA matrix effectively improves the initial degradation temperature of PUA nanocomposites. Herein, the surface covalent functionalization of PZS nanotubes will provide a promising method to fabricate the UV-curable polymer nanocomposite coatings with reinforcing performance.Acknowledgements
The work was financially supported by the National Basic Research Program of China (973 Program) (No. 2012CB719701), the National Natural Science Foundation of China (No. 21374111) and (No. 51203146), the Science and Technology Program of Guangzhou, China (No. 2014J4100174), the Fundamental Research Funds for the Central Universities (WK2320000032).References
- J. V. Crivello and E. Reichmanis, Chem. Mater., 2013, 26, 533–548 CrossRef.
- C. Ingrosso, C. Esposito Corcione, R. Striani, R. Comparelli, M. Striccoli, A. Agostiano, M. L. Curri and M. Frigione, ACS Appl. Mater. Interfaces, 2015, 7, 15494–15505 CAS.
- H.-D. Hwang, C.-H. Park, J.-I. Moon, H.-J. Kim and T. Masubuchi, Prog. Org. Coat., 2011, 72, 663–675 CrossRef CAS.
- H. Xu, F. Qiu, Y. Wang, W. Wu, D. Yang and Q. Guo, Prog. Org. Coat., 2012, 73, 47–53 CrossRef CAS PubMed.
- D. Kim, M. Lim, I. Kim, J. Seo and H. Han, Prog. Org. Coat., 2014, 77, 1045–1052 CrossRef CAS PubMed.
- T. Zhang, W. Wu, X. Wang and Y. Mu, Prog. Org. Coat., 2010, 68, 201–207 CrossRef CAS PubMed.
- J. Guan, Y. Song, Y. Lin, X. Yin, M. Zuo, Y. Zhao, X. Tao and Q. Zheng, Ind. Eng. Chem. Res., 2011, 50, 6517–6527 CrossRef CAS.
- S. Liang, N. M. Neisius and S. Gaan, Prog. Org. Coat., 2013, 76, 1642–1665 CrossRef CAS PubMed.
- P. J. Peruzzo, P. S. Anbinder, O. R. Pardini, J. Vega, C. A. Costa, F. Galembeck and J. I. Amalvy, Prog. Org. Coat., 2011, 72, 429–437 CrossRef CAS PubMed.
- D. Cai, J. Jin, K. Yusoh, R. Rafiq and M. Song, Compos. Sci. Technol., 2012, 72, 702–707 CrossRef CAS PubMed.
- X. Wang, Y. Hu, L. Song, H. Yang, W. Xing and H. Lu, J. Mater. Chem., 2011, 21, 4222–4227 RSC.
- S. K. Yadav and J. W. Cho, Appl. Surf. Sci., 2013, 266, 360–367 CrossRef CAS PubMed.
- B. Yu, X. Wang, W. Xing, H. Yang, L. Song and Y. Hu, Ind. Eng. Chem. Res., 2012, 51, 14629–14636 CrossRef CAS.
- H. Liu, J. Song, S. Shang, Z. Song and D. Wang, ACS Appl. Mater. Interfaces, 2012, 4, 2413–2419 CAS.
- H. R. Allcock and N. L. Morozowich, Polym. Chem., 2012, 3, 578–590 RSC.
- Y. Hu, L. Meng, L. Niu and Q. Lu, ACS Appl. Mater. Interfaces, 2013, 5, 4586–4591 CAS.
- W. Jin, L. Yuan, G. Liang and A. Gu, ACS Appl. Mater. Interfaces, 2014, 6, 14931–14944 CAS.
- X. Liu, Z. Tian, C. Chen and H. R. Allcock, Macromolecules, 2012, 45, 1417–1426 CrossRef CAS.
- N. L. Morozowich, J. L. Nichol and H. R. Allcock, Chem. Mater., 2012, 24, 3500–3509 CrossRef CAS.
- T. Modzelewski and H. R. Allcock, Macromolecules, 2014, 47, 6776–6782 CrossRef CAS.
- H. R. Allcock, E. C. Kellam and M. A. Hofmann, Macromolecules, 2001, 34, 5140–5146 CrossRef CAS.
- T. Pan, X. Huang, H. Wei, W. Wei and X. Tang, Macromol. Chem. Phys., 2012, 213, 1590–1595 CrossRef CAS PubMed.
- I. Teasdale and O. Brüggemann, Polymers, 2013, 5, 161–187 CrossRef PubMed.
- L. Zhu, Y. Zhu, Y. Pan, Y. Huang, X. Huang and X. Tang, Macromol. React. Eng., 2007, 1, 45–52 CrossRef CAS.
- Z. Chen, J. Zhang, J. Fu, M. Wang, X. Wang, R. Han and Q. Xu, J. Hazard. Mater., 2014, 273, 263–271 CrossRef CAS.
- J. Fu, X. Huang, Y. Huang, J. Zhang and X. Tang, Chem. Commun., 2009, 1049–1051 RSC.
- J. Fu, Q. Xu, J. Chen, Z. Chen, X. Huang and X. Tang, Chem. Commun., 2010, 46, 6563–6565 RSC.
- L. Zhu, Y. Xu, W. Yuan, J. Xi, X. Huang, X. Tang and S. Zheng, Adv. Mater., 2006, 18, 2997–3000 CrossRef CAS PubMed.
- Y. Zhu, X. Huang, W. Li, J. Fu and X. Tang, Mater. Lett., 2008, 62, 1389–1392 CrossRef CAS PubMed.
- P. Zhang, X. Huang, J. Fu, Y. Huang, Y. Zhu and X. Tang, Macromol. Chem. Phys., 2009, 210, 792–798 CrossRef CAS PubMed.
- X. Zhang, X. Huang and X. Tang, New J. Chem., 2009, 33, 2426–2430 RSC.
- M. Wang, J. Fu, D. Huang, C. Zhang and Q. Xu, Nanoscale, 2013, 5, 7913–7919 RSC.
- J. Fu, X. Huang, Y. Zhu, Y. Huang and X. Tang, Appl. Surf. Sci., 2009, 255, 5088–5091 CrossRef CAS PubMed.
- J. Zhang, X. Huang, J. Fu, Y. Huang, W. Liu and X. Tang, Mater. Chem. Phys., 2010, 121, 511–518 CrossRef CAS PubMed.
- X. Gu, X. Huang, H. Wei and X. Tang, Eur. Polym. J., 2011, 47, 903–910 CrossRef CAS PubMed.
- C. H. See, O. M. Dunens, K. J. MacKenzie and A. T. Harris, Ind. Eng. Chem. Res., 2008, 47, 7686–7692 CrossRef CAS.
- Z. Chen, J. Fu, M. Wang, X. Wang, J. Zhang and Q. Xu, Appl. Surf. Sci., 2014, 289, 495–501 CrossRef CAS PubMed.
- J. Fu, X. Huang, Y. Huang, Y. Pan, Y. Zhu and X. Tang, J. Phys. Chem. C, 2008, 112, 16840–16844 CAS.
- S. Zhang, J. Jiang, C. Yang, M. Chen and X. Liu, Prog. Org. Coat., 2011, 70, 1–8 CrossRef CAS PubMed.
- C. G. Robertson and M. Rackaitis, Macromolecules, 2011, 44, 1177–1181 CrossRef CAS.
- A. K. Kaushik, P. Podsiadlo, M. Qin, C. M. Shaw, A. M. Waas, N. A. Kotov and E. M. Arruda, Macromolecules, 2009, 42, 6588–6595 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra12264c |
| This journal is © The Royal Society of Chemistry 2015 |