Geometrical modifications and tuning of optical and surface plasmon resonance behaviour of Au and Ag coated TiO2 nanotubular arrays†
Dhiman Bhattacharyyaa,
Prashant K. Sarswat*a,
Maidul Islamc,
Gagan Kumarc,
Mano Misraab and
Michael L. Freea
aDepartment of Metallurgical Engineering, University of Utah, Salt Lake City, UT 84112, USA. E-mail: prashant.sarswat@utah.edu
bDepartment of Chemical Engineering, University of Utah, Salt Lake City, UT 84112, USA
cDepartment of Physics, Indian Institute of Technology Guwahati, Guwahati 781039, India
First published on 12th August 2015
Abstract
A protocol for surface structuring and shape modifications of anodized TiO2 nanotubes by controlling the electric field is presented. The shape and size control of nanotubes is essential in order to achieve more control over optical and electronic properties of nanotubes such as effective band gap, light absorption capabilities, photoelectrochemical response, Raman properties, as well as appearance of the localized states. A surface plasmon resonance (SPR) study was performed using these newly developed nanotubes. It was observed that noble metal treated nanotube assemblies exhibit a ‘slow photon effect’ and plasmon-exciton interaction. Such a ‘slow photon’ effect is highly advantageous to improve photoelectrochemical (PEC) performance and quantum efficiency if plasmonic PEC activity is coupled with it in the SPR region. The FDTD simulations of tilted nanotubes suggest that the maximum surface field confinement can be realized for a lower bend angle of 8° and this confinement diminishes when inclination of the nanotubes increases further.
1. Introduction
Shape and size dependent optical responses of photonic crystals1 are a great advancement that was achieved 25 years ago. This development revolutionized the previously established view pertaining to the ‘immutable and fixed behavior of material, whilst interacting with electromagnetic waves’. Photonic crystals that were fabricated precisely, exhibited an adequate control and desired manipulation of the flow of light.1 Therefore, titania nanotube arrays (TNA) constitute an important material category consistent with this observation. This can be attributed to the fact that precise control over the shape and size of nanostructures, along with varied morphology can be obtained, whilst negating the use of techniques such as lithography or FIB machining. Surprisingly, TNA has not been well investigated from this point of view. The versatility of TiO2 has been predominantly observed due to its wide band gap and a superior charge diffusion coefficient. The ability to synthesize, and the applications of, one-dimensional nanostructured TiO2 thin films, such as nanorods, nanotubes, and nanoforests are unprecedented. The crystalline nanobranches of nanoforest type TiO2 morphology act as preferential electron pathways resulting in higher charge collection efficiency, compared to disordered TiO2 nanoparticles that often suffer higher electron recombination problems. However, in a constant effort to provide enhanced charge transport capabilities and a large surface area, the morphology of TiO2 has often been tweaked to meet certain requirements, including improved photoconversion efficiency (PCE).2–5Nanorods or nanoforest like morphologies do not possess high internal surface area. However, with a TiO2 nanotube based morphology, the internal surface area concern can be mitigated.6–10 Although these structures are of technological importance, others such as tapered, conical or other specially designed nanostructures have also drawn significant attention due to their enhanced charge transport capabilities and other desirable properties in comparison to their regular shaped counterparts. The evanescent field properties in a tapered optical fiber illustrated that the manipulation of nanoparticles through such evanescent fields is feasible and such a method can be utilized for sorting of metallic nanoparticles, selective optical trapping, or transporting nanocrystals.11 Similarly, biconical tapered silica fibers have shown great potential in sensing applications.12 It was observed that modified geometries possessed increased sensitivity due to the strong interaction of the analyte, enabled by an evanescent field caused by tapering. The shape of the depth profile of photon absorption and carrier generation partially governs the efficiency of TNA based dye sensitized solar cells, in many cases.13 Experimental results and finite difference time domain (FDTD) assisted simulations suggested a relatively fast electron dynamics in conical tubes as compared to cylindrical tubes, although total light absorption was similar for both cases. Base tapered and conical shaped InP based nanostructures exhibited enhanced broad band and omnidirectional light absorbance in comparison to their regular cylindrical shaped counterparts.14 Tapered nanotips can be used to deliver laser beams of nanoscale cross sections for fluorescence studies in single cells.15 Other low cost integrated optical sensors with tapered geometry have been used for monitoring of air pollution and species concentrations in various environments.16 Similarly, tapered fiber optic evanescent wave sensors have been fabricated and modified for other applications such as hydrogen gas sensor,17 liquid hydrocarbon sensor,18 and pH sensor.19 Tapered carbon nanotubes (TCNTs) have been studied for their field emission properties.20 It was concluded that TCNTs are effective field emitters due to their high aspect ratio and the special tapered tip with small radius of curvature. Tapered nanocapillaries on porous anodic alumina were fabricated through multistep anodization wherein the diameter of the nanopore was varied.21 Mor et al. fabricated tapered TNA and mentioned their applications in biofiltration membranes, biomolecular traps, photocatalytic and sensing materials.22 Y-branched titania nanotubes have been synthesized using a multistep sonoelectrochemical anodization method.23 It was observed that Y-branched nanotubes' visible light absorbance is more efficient than regular 1D nanotubes.23 Elongated Y-shaped PFO-DBT nanotubes have also shown enhanced light absorption with less light scattering.24 Overall, this passage of discussion indicates an urgent need to evaluate the shape dependent optical and vibrational properties in detail, for such nanotube arrays.
Thus, in an effort to further understand and improve the light absorption capabilities, self-aligned and highly ordered TiO2 nanotubes of varying morphologies were synthesized using a one-step, fluorinated ethylene glycol solution based anodization process. The architectural modifications of the nanostructured titania nanotube photoanode enable the possibility of SPR enhancement and their better usage efficiency over a broader wavelength spectrum involving plasmonic nanoparticles. One dimensional photonic crystals possess a spectral range of large reflectivity, known as the stop band, which can be modified by altering the size or shape of the structural repeat unit as per Bragg's law.25 In TNA based ordered photonic crystal with periodic dielectric structure, slow photons are observed at a frequency close to the photonic stop band edge. This is due to a fact that at the red edge of the photonic stop band, a slow photon propagates with a reduced group velocity (owing to the high refractive index of titania).25 Hence, the coupling of the slow photon region with the SPR region for efficient light harvesting in the UV-visible region will be demonstrated in this report. Apart from the optical properties, TNA of various morphology were characterized for their structural, vibrational, and plasmonic properties including FDTD simulations.
2. Experimental
The titanium foils of G1 grade and 0.1016 mm thickness were purchased from ESPI Metals (Ashland, OR) and cut into coupons of 1.5 cm × 1.5 cm. These titanium coupons were polished with 400-b grit emery paper to remove surface oxides. Subsequently, these coupons were degreased by ultrasonication in acetone–ethanol (50% v/v each) mixture for 30 minutes followed by rinsing with deionized water. The cleaned coupons were electrochemically anodized in a fluorinated ethylene glycol solution (0.5 wt% NH4F + 3 wt% H2O) in a Teflon beaker with platinum foil as the counter electrode at room temperature.In order to obtain the straight vertically oriented nanotubes, the electrochemical anodization was performed at a constant potential of 30 V (Agilent E3647A dc power source) for one hour under constant stirring at 60 rpm. Ordered arrays of tapered (or conical) titania nanotubes were obtained by keeping voltage constant at 20 V for 5 minutes to allow the formation of the TiO2 barrier layer, followed by linearly ramping the voltage to 60 V over a period of 45 min at the rate of 0.88 V min−1. It was followed by a dwell time of 10 minutes. Dumbbell shaped titania nanotubes were produced using a constant voltage of 60 V that was maintained for 10 minutes followed by ramping down to 20 V over 20 minutes at the rate of 2 V min−1, then ramping up from 20 V to 60 V at 2 V min−1 and finally keeping it constant at 60 V for another 10 minutes. In all the above the cases, a distance of 3 cm between the Pt cathode and the anode was constantly maintained during the anodization process and the stirring was continued at 60 rpm.
In order to synthesize tilted TiO2 nanotubes, the experimental set-up was modified. In the setup described in the preceding paragraph that involves a cathode and an anode, a Synchron 630 motor (110 V, 60 Hz, 4 W) was attached to the anode to facilitate gradual rotation of the anode. An initial distance between the anode and the Pt cathode was maintained at 3 cm, though the distance changed as the anode rotated along its vertical axis (see Fig. 1). Anodization was carried out at a constant 60 V potential for 1 hour. Magnetic stirring was avoided in this case to prevent rupture of the tilted nanotubes. After one hour, the anodized titania coupon had undergone an angular rotation of about 10 degrees.
A tree shaped nano-tubular structure was obtained when the anodization potential was suddenly changed. Initially, a constant voltage of ∼20 V was maintained for ∼3 minutes and sudden alteration in voltage was done. The voltage was ramped up to 60 V over a period of 15 minutes at the rate of 2.66 V min−1 and then maintained at 60 V for the remaining 42 minutes. Such a rapid potential change during the initial phase, when the barrier layer was still developing, resulted in nanograss-like structures protruding out of the nanotubes.
For all the above cases, after anodization, the coupons were rinsed with isopropanol and sonicated in DI water for 15 seconds to remove the debris and glycol solution from the titania nanotube surface and subsequently, they were dried overnight at 110 °C under vacuum. Further, the anodized coupons were calcined in air using a box furnace set at temperature ∼500 °C (25 °C to 500 °C over 3 h) for 2 hours.
After synthesis of various nanoarchitectured TNA arrays, gold and silver nanoparticles were immobilized on the surface using a dip coating functionalization technique. Gold nanoparticles/nanocrystals were synthesized using reduction of HAuCl4.26,27 Ag nanoparticles were obtained from Nanopowders Industries (Utah) Ltd. To observe the effect of the change in morphology on optical and plasmonic properties, systemic characterization was performed using UV-Vis spectroscopy, Raman spectroscopy, scanning electron microscopy (SEM), X-ray photoelectron spectroscopy, and energy dispersive spectroscopy (EDS).
The various morphologies of the synthesized nanotubular arrays were characterized using a field emission scanning electron microscope (Hitachi S-4800 SEM) maintained at ultra-high vacuum. An intense electron beam from a tungsten filament at 3 kV accelerating voltage and 15 μA emission current setting was used for imaging at very high magnification (∼90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×). Energy dispersive X-ray spectroscopy (EDS) mapping was achieved using an Oxford EDX detector attached to the SEM. The EDS studies were performed at 20 kV accelerating voltage and high probe current over a 25 μm × 25 μm sample area.
000×). Energy dispersive X-ray spectroscopy (EDS) mapping was achieved using an Oxford EDX detector attached to the SEM. The EDS studies were performed at 20 kV accelerating voltage and high probe current over a 25 μm × 25 μm sample area.
To further confirm the presence of gold and to determine its oxidation states, X-ray photoelectron spectroscopy (Kratos Axis Ultra DLD model) was used. A monochromatic X-ray excitation source of Al Kα radiation was used while vacuum in the analysing chamber was of the order of ∼10−10 Torr. CasaXPS software was used for peak fitting. Charging effects were corrected using the C1s line at 284.6 eV as an internal reference. Shirley-type background was subtracted from the spectra.
UV-Vis spectroscopy was performed using a UV-3600 UV-Vis-NIR spectrophotometer (Shimadzu, Kyoto, Japan) equipped with UV-Probe software to illustrate absorbance and reflectance properties. An external 3-detector system was used while the slit width was maintained at 32 nm and the wavelength was varied from 800 nm to 300 nm with a 1 nm step size.
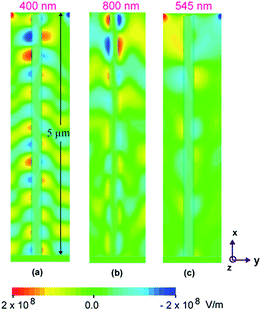
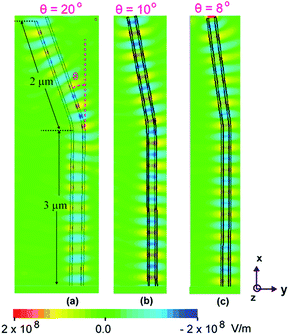
In order to have an appreciation of the effect of geometry on the electromagnetic field profile in the fabricated nanotube structures under the incidence of visible light of certain wavelength, simulations have been performed for straight nanotubes at three different wavelengths of (a) 400 nm (b) 800 nm, and (c) 545 nm respectively. A finite element time difference solver was utilized to calculate the field profiles in the xy plane of the nanotubes by an incident light from the top. In simulations, a waveguide port with polarization in the y-direction to approximate the incident polarized light of certain wavelength, was utilized. The inner and outer diameters were assumed 80 nm and 90 nm, respectively, corresponding to the experimentally fabricated samples. TiO2 nanotubes with total length of 5 μm are approximated with a material of dielectric constant of 2.42. Further, to investigate the effect of tilt geometry of the titania nanotubes on light absorbance, fabricated single nanotube structure under the incidence of visible light was simulated with different bend angles of 8°, 10° and 20° at a fixed wavelength of λ = 400 nm.
3. Results and discussion
3.1 Morphological examination
SEM images of different nanoarchitectured titania nanotubular arrays are illustrated in Fig. 2. Anodization at constant potential resulted in vertically oriented highly ordered straight nanotubes of constant diameter of 85 nm (Fig. 2a). Tapered nanotubes obtained due to ramping voltage conditions are ∼80 nm in diameter (in bottom, constant-voltage anodization regime) but show a conical shape of regularly decreasing diameter (in top, ramping-voltage anodization regime) of ∼40 nm (Fig. 2b). Dumbbell shaped nanotubes can be grown as observed in Fig. 2c, when inverse triangular voltage signal was applied. It can be seen that there is difference in diameter between center and the end of nanotube. The diameter near the center region ∼50 nm while that of the end of the nanotube ∼85 nm. Similarly tilted nanotubes are possible (see Fig. 3a and b); they exhibit either clockwise or counter clockwise rotation (or tilt) depending on direction of rotation of the synchronous motor. The titled nanotubes produced with the setup in this study are similar to those grown on curved or patterned surfaces.28 It can be seen that diameter of nanotube is ∼82 nm which remains nearly constant along length of the nanotubes, while a maximum tilt angle of ∼8° is achieved during ∼10 degree angular rotation of the Ti anode. For nanograss type tube morphology, the diameter towards the bottom of the tube was ∼78 nm while the thickness of the grass-like nanostructures was of the order of a few (8–10) nanometers (see Fig. 4). SEM micrographs were obtained after functionalization of the TNA with gold and silver nanoclusters. The size of Ag nanoparticles ranged from 80–100 nm, Au nanoparticles were about the same size as well. The Au nanoclusters were 200–450 nm in size. The diameter of very small Au nanoparticles was ∼10–20 nm. EDS mapping confirmed the presence of gold and silver nanoparticles coated on the TNA surface (see Fig. 5). | ||
| Fig. 3 Effect of bending of electric field lines on the growth direction of TNA, due to (a) anticlockwise and (b) clockwise rotation, in the case of tilted nanotube morphology. | ||
Other information related to elemental characterization (XPS data), chemical, and electronic states of surface materials have been included in ESI.†
3.2 Mechanism of shape control
The mechanism describing the formation of the various electric field-driven shapes of titania nanotubular arrays can be attributed to a sequence of key processes. The titania nanotubes are essentially formed by an etching process where the balance of the TiO2 dissolution and oxidation processes leads to the growth of nanoporous structures. The TiO2 barrier layer dissolves according to the following reaction:| TiO2 + 6F− + 4H+ → TiF62− + 2H2O | (1) |
Hence, it is evident that the fluoride ions in the solution react with the TiO2 in acidic environment to cause etching and is supposedly the dominant reaction resulting in the formation of the nanotubular structure. The dissolution of the titania in the fluoride rich solution is an integration of two distinct phenomena: chemical dissolution and electric field assisted dissolution. Hence, both solution stoichiometry and applied potential are considered important for the formation of a nanotubular morphology.
First, it would be appropriate to describe the mechanism for straight nanotubular morphology. During the overall anodization process (at a constant voltage), total change in F− ions consumption by the chemical dissolution, is very low and doesn't contribute much from a rate calculation standpoint. Hence, it can be assumed that the fluoride concentration remains nearly constant during the course of the etching reaction. Consequently, it is reasonable to assume that the etching rate is constant. Thus, the rate of change of the nanotube diameter is essentially independent of time. As a result, the nanotube diameter at the start of the process (that is mainly governed by the anodization voltage) remains constant throughout the process, resulting in formation of straight nanotubes.
Variation in nanotube diameter occurs due to rapid or gradual change in the applied anodization potential, which can cause a disturbance in the concentration of the fluoride ions near the anode. It has been mentioned that during a linear increase in voltage, the growth dynamics is directly related to instantaneous voltage.22 Increasing the voltage can cause an increase in the instantaneous concentration of the fluoride ions near the anode leading to enhanced inward movement of the barrier layer. This results in an increase of the tube diameter. The diameter at the bottom of the nanotube is determined by the final applied voltage. Conical or tapered nanotube morphology can be considered as a cone on top of a straight cylinder. Thus, the dwell time at the final voltage determines the length of the straight portion of the nanotube. It had also been mentioned that the residence time at the final voltage plays a significant role in determining the diameter of the tapered end of the nanotubes.22 It is worth noting that etching caused by chemical and field assisted dissolution of the surface layer of the nanotube at higher voltages differs from that obtained at lower initial voltages.
For chemical dissolution29 of titania, the reaction of F− ions with the TiO2 is limited by the surface area of contact. Diffusion of F− into the lattice of titania is extremely slow and does not warrant consideration here from a practical point of view. Thus, the rate of change of the nanotube diameter (or dissolution of the titania) can be determined by considering that the rate of dissolution29 depends on a dissolution constant (equivalent to mass transfer coefficient), surface area of transfer and the concentration of the fluoride ions causing the dissolution. This can be expressed as:12
 | (2) |
But, RTNT is the radius of nanotube, RTNT = DTNT/2; eqn (2) can be written as:
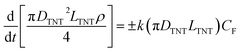 | (3) |
 | (4) |
 | (5) |
 | (6) |
This suggests that the titania nanotube diameter can vary linearly over time as a result of chemical dissolution. At the oxide-solution interface, metal and oxygen-containing ions are transferred between the oxide and the solution phases. Due to this ion-transfer reaction, there is an interfacial potential drop. In addition, an equilibrium potential difference exists due to the difference in oxygen concentration in the oxide and the solution. The difference between the interfacial and the equilibrium potential drop gives a measure of the overpotential (η where, η = E − Eeq). Zero overpotential would mean that the film thickness remains constant with time due to no net transfer of oxygen across the interface; however, interfacial metal ion transfer is not in equilibrium and there is a steady-state current due to Ti4+ dissolution. This overpotential kicks in Butler–Volmer reaction kinetics which takes into account the anodic and the cathodic reactions and the current density depends on the field (or overpotential to be precise). When the overpotential is not too large, Butler–Volmer equation controls the kinetics (which is purely activation controlled current) and this condition might exist during the initial nucleation period where the growth of the cylindrical nuclei is the predominant mechanism. But as the voltage is varied, the overpotential increases. The critical nucleation size is inversely proportional to the square of the overpotential. Due to large overpotentials, the nanotube nucleation size is small (of the order of ∼nm) and this is the initiation point of 1D geometry of nanotubular array. Also, at large overpotentials, the effect of Butler–Volmer kinetics is limited and mass transport process is predominant. According to the reaction stoichiometry, concentration of metallic Ti4+ ions in the solution is one sixth that of the fluoride ion concentration. The concentrations of fluoride ions CF (as well as Ti4+ ions) near the anode changes due to the change in the applied anodization potential, under the given experimental conditions.30,31 It is due to the field assisted dissolution29 that can be represented by a non-linear state-space model. This model relates the dissolved fluoride ion concentration to cell voltage, it can be represented in the mathematical form as:12,29
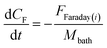 | (7) |
| vcell = f(CF, θ) | (8) |
| vcell = f(CF, ACD, θ′) | (9) |
The combined effect of the chemical and field assisted dissolution on the nanotubes diameter as the voltage was varied over time can be validated from experimental results. The dimensions of the nanotubes obtained from visual examination of the SEM images, were used to correlate the rate of change of nanotube diameter to the voltage ramp rate. It was observed that the average diameter of the titania nanotubes at 60 V was around 80 nm. The plot of the rate of change of tube diameter against the voltage ramp rate exhibits a linear relationship as expected from the proposed mathematical model based on chemical dissolution (see Fig. 6a). This model validation is based on the assumption that the rate of change of fluoride ion concentration near the anode due to the field effect is at par with the voltage ramp rate. In reality, this might not be the case and it can contribute to non-linearity. The dissolution model as stated in eqn (6) was independently verified for the tapered and dumbbell morphologies. A plot of DTNT/Di vs. time yielded the dissolution constant, ‘K’. The calculated value (from the slope) of ‘K’ for tapered nanotubes is 0.021 min−1 (positive slope indicating gradual increase in dia.) and that for dumbbell shaped nanotubes is 0.022 min−1 (negative slope indicating gradual decrease in dia.). The points given in the plots shown in Fig. 6b and c are data points and the scale bar corresponds to the SEM images given in Fig. 2. For tapered nanotubes, the voltage was gradually ramped up resulting in an increase in diameter; thus, the slope is positive. For dumbbell shaped nanotubes, the voltage was ramped down resulting in a decrease in diameter followed by voltage ramp up. The model plots the change in diameter when the voltage was decreased; thus, the negative slope. During the nanotube formation, for both tapered and dumbbell shaped morphologies, the etching of the titania barrier layer is a gradual process and is thus, the rate limiting step. The values of the dissolution constant are almost equal for both the morphologies and are testament to the fact that chemical and field assisted dissolution are the dominant mechanisms.
In the case of nanoforest type morphology, unsteady state growth kinetics exists during the nanoforest growth regime which is mostly field dominated. Thus, the dissolution constant cannot be calculated. Steady state growth is achieved once the voltage becomes steady, which dictates the diameter of the nanotube portion. The length of the nanotube portion is governed by the dwell time under steady voltage conditions.
3.3 Structure and shape dependent vibrational properties
Raman spectroscopy of TiO2 NTA substrates were carried out in order to investigate phase purity (Fig. 7). A R3000 QE Raman spectrometer was utilized in backscattering mode. Raman measurements were performed using a laser with a wavelength of ∼785 nm and a power of ∼95 mW. The wavelength stability and drift were examined using standard silicon sample measurements and it was observed to be less than 1 cm−1 over a 12 h period. Raman spectra of straight, conical, tilted, and dumbbell shaped nanotubes show three distinct peaks that correspond to anatase phase of TiO2. Such three modes were obtained at ∼395 cm−1 (B1g), 516 cm−1 (A1g) and 640 cm−1 (Eg), respectively and they correspond well with earlier reported Raman spectra of TiO2.32 It is interesting to observe that Raman intensity of irregular shaped titania nanotubes are stronger compared to straight or vertically grown TNT arrays. One possible explanation of such behaviour can be attributed to the difference in thickness of TNT arrays. Another potential cause of different Raman scattering is the effect of polarization of incident laser with respect to nanotube axis. Polarization dependent Raman scattering have been performed for carbon nanotube (CNT) films.In situ Raman measurements were performed to correlate growth rate with chiral angles and their subsequent effect on Raman intensity.33 It was observed that these parameters alter Raman behaviour. Similarly, in another report it was concluded that torsion strain greatly affects the Raman response of SWCNT.34 Hence, it can be said that strain, polarization direction, and growth direction have great effects on the Raman response of TNA, apart from the thickness of the TNA film.
3.4 Optical properties, plasmonic resonance, and FDTD simulations
Band gap measurements were performed using UV-Vis spectroscopy data (see ESI†), and it was observed that there is an effect on band gap, causing a lowering of band gap from 3.25 eV to 3.15 eV due to shape modifications. The narrowing of band gap energy had been observed in zigzag shaped SWCNTs too. An interesting similarity between CNT and TNA (TiO2) is that both have large elastic constants35 (TNA: 100–500 GPa; CNT: 1 TPa).35,36 The stress coefficient of band gap, which is defined as the ratio of stress deformation potential to elastic constants, is relatively small in titania mainly due to large elastic constants. Particularly, in the case of anatase phase titania nanotube, Young's modulus is expected to be relatively small along its vertical axis and as a result, any small magnitude of stress applied or developed in the weakest crystal direction may significantly affect the bond length and bond angles.Various nanoarchitectured morphologies of titania nanotubes give rise to multiple stress locations along the axis due to the anomaly in the nanotubular geometry relative to straight ones. A very similar assumption was validated from effective band gap narrowing of anatase TiO2 due to strain along a soft direction.37 Thus, narrowing of band gap energy can be attributed to localized shape modification causing increased curvature along the circumference of a deformed cross section.38 The stress due to bending causes the alteration in bond length and angles, that causes a change in energy band gap, as can be seen in case of anatase TiO2 nanotubes.37
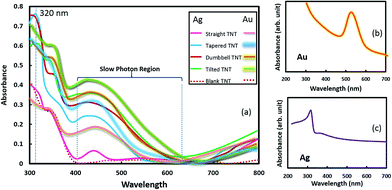
The optical absorbance (see Fig. 8a) of the Au and Ag nanoparticles coated TiO2 nanotubes of various geometries increased sharply at a wavelength ∼420–400 nm and this corresponds to the strong intrinsic light absorption of rutile and anatase phase TiO2 (Eg ∼ 3–3.2 eV). Systematic examination of the absorbance plots indicates an interaction between surface plasmon of Au nanocrystals and TiO2 excitons that originates from the exciton-dipole modification, mainly due to surface plasmon local electromagnetic modes.39 Dipole–dipole interaction is not very significant for smaller size Au crystal but can be prominent for larger size Au crystals, as evidenced in SEM images. The broad absorption region between 400–600 nm can be categorized as the ‘slow photon region’,25 and this slow photon effect is more pronounced in tapered and tilted nanotubes and less evident in straight nanotubes.
In such a substrate, slow photons are observed at a frequency close to the photonic stop band edge. This is due to the fact that at the red edge of the photonic stop band, slow propagation of the light photons takes place with a reduced group velocity owing to the high refractive index of titania. It has been demonstrated that photon density is higher in such a region, when measured in high refractive index medium compared to in air.25 It is important to note that TiO2 has higher refractive index ∼ 2.42, hence, the slow photon region and the SPR region (SPR absorption band of freely dispersed gold and silver nanoparticles was found to be ∼525 nm and 315–320 nm (see Fig. 8b and c) respectively, and corroborates well with reported literature values) will be merged (overlapped) due to strong interaction between SPR effect of Au particles and localized photons. This can be attributed to the fact that photon density in the slow photon region is larger in the high refractive index part (TiO2 nanotubes) compared to air voids. Such a ‘slow photon’ effect is advantageous to improve photoelectrochemical (PEC) performance and quantum efficiency, if plasmonic PEC activity is coupled with slow photon effect in SPR region. Interestingly, Bragg reflection that causes some negative effect in performance, can also be dealt with as seen in different absorbance plots, due to shape modifications. Here it should be noted that for Ag nanocrystals coated TiO2 nanotubes, SPR region of Ag is outside the slow photon region of TiO2 nanotubes.
In other words, the SPR region of Ag is towards the blue edge of the photonic stop band while the slow photon effect is towards the red edge. Such an unfavorable synergistic combination of the two photoactive components viz. titania nanotubes photonic crystals and the plasmonic Ag nanostructures, suppress the plasmonic photoactivity in the visible region. The SPR resonant activity of the plasmonic nanostructures can be altered by varying the geometry of the photonic crystal. Fig. 9 shows the logarithm of relative absorbance of Au and Ag treated TiO2 nanotubes with respect to reference substrates (tapered, tilted, or dumbbell; without any metallic nanocrystals coating). For tapered TiO2 nanotubes, the absorbance peaks were observed at ∼569 nm whereas for tilted and dumbbell shaped nanotubes, the absorbance intensity was highest at wavelength ∼659 nm, irrespective of type of nanocrystals coating. Modified Maxwell-Garnet approach was utilized to model SPR behavior of TiO2–Au composite.40 Using this model, absorption spectra were simulated that suggest a strong absorption peak at ∼625 nm. However, Bragg's reflection and Rabi oscillation41 conditions are expected to be different for TiO2 nanotube arrays and bulk TiO2 (or TiO2 thin film), due to the difference in morphology. This may be one of the reasons that causes a shift in the absorbance peak. In addition, the favorable position of the photonic stop band towards the near infrared region in the tilted and dumbbell shaped nanotubes contributes to the red shift in the slow photon effect for these geometries. It is clear from Fig. 8 and 9 that tilted TiO2 nanotubes are more effective absorbers when used in combination with metallic (Ag, Au) nanocrystals. It can be attributed to diminution of specular reflectance at the interface and an increase of the optical path length in the nanostructure by multiple photon scattering.14
The light propagation through TiO2 nanorods and nanotubes have been previously investigated using FDTD simulations.42 Due to high contrast between the refractive index and permittivity of TiO2 and its surrounding medium, such nanotubular architecture can be categorized as a ‘potential photonic material’. Such a combination also facilitates the utilization of surface resonances due to interaction of light with the nanotubular morphology. In the work presented here, the titania nanotube wall thickness was consistent between 8–10 nm for all studied nanoarchitectured morphologies and thus, can be classified as very thin walls. Such thin walls act as subwavelength type Rayleigh scattering medium wherein the wavelength of light is much larger than the wall thickness.42 In addition to thin tube walls, the various morphologies such as tapered, dumbbell shaped and tilted TiO2 nanotubes will aid in further light scattering, which is beneficial from a light harvesting standpoint. Thick walled nanotubes with a distinct hollow annulus can accommodate guided modes of plane polarized electromagnetic radiation through the walls. On the contrary, for sub 10 nm thin walled titania nanotubes, accommodation of these guided modes are difficult and thus, exhibit weaker resonance. The resonance behavior can be enhanced by doping plasmonic materials on TiO2 such as gold or silver nanoparticles, as has been observed. It is also interesting to note though that when thin walled TNA is exposed to plane wave light illumination, the resultant optical field comprises waveguides from the array which are characterized by a combination of slow and fast phase velocity modes, arising from the high permittivity nanotube wall medium and low permittivity air medium present inside the hollow nanotubes, respectively.42 Although the fast waveguide modes are predominant and strong, the slow waveguide mode contributes to the slow photon effect, as illustrated through our results.
For FDTD simulations, tetrahedral shaped grids of size ∼λ/10 were used. For these simulations, a perfectly matched layer boundary condition was applied, in order to avoid reflections that can interfere with actual surface field guided by nanotube. A waveguide port at a distance of ∼200 nm from the top was utilized, that acts as a plane wave source that resembles actual experiments. The FDTD simulation of the specular absorbance as a function of wavelength for straight titania nanotubes is shown in Fig. 10 for incident light polarized along the y-direction. Although, the titania nanotubes were grown on Ti substrate, it is presumed that the predominant light adsorption is due to the titania nanotubular array only and thus, the simulated absorbance of the nanotube, ignoring the Ti substrate, is presented. The electric field distribution around the nanotubes provides information about the light absorption by the semiconductor material and is determined by the y-component or the dominant component of the electric field. This y-component is oriented parallel to the polarization vector of the incident wave. The FDTD simulations reveal that as expected, the absorbance in the titania nanotube layer is maximum at λ = 400 nm. For wavelengths longer than the electronic bandgap of TiO2, the absorbance is very low or negligible. The effect of nanotube tilt geometry (see Fig. 11) on visible light absorbance was investigated too. It was observed that light was efficiently coupled to waveguide modes in the tilted TiO2 nanotube region giving rise to high internal fields. One may note from the field profiles that it is confined along the designed nanotube structures signifying the propagation of surface electromagnetic wave along the nanotube.
As the bending angle was increased, the surface field confinement decreased as evident in Fig. 11. The maximum field confinement is observed for a bend angle of 8° and this corroborates well with experimentally synthesized tilted titania nanotubes having a tilt of 8–10°.
The electromagnetic wave propagation profiles of a single nanotube can be extended to an infinite array of nanotubes. Such a nanotubular array upon exposure to a plane wave illumination will yield an optical field comprising slow waveguide modes confined to the nanotube walls with a higher dielectric constant and the faster modes in the surrounding medium (air) with a lower permittivity. Interference between slow and fast modes result in intensity oscillations as observed in our FDTD simulations too.
4. Conclusions
In summary, various nano-architectured titania nanotube arrays (TNA) were synthesized by adequate control of mass transfer and electric field. A significant alteration in tube diameter was observed upon the application of a gradient of anodization voltage. Hence, continuous control over the anodization potential can provide a nanotube of desired longitudinal cross section. It was observed that an increase in anodization voltage, causes an enhanced rate of oxidation and field assisted dissolution. Thus, based on the inference derived from experimental evidence, it is important to note that the nanotube pore entrance remains unaffected by the electric field assisted dissolution whereas the electric field distribution in the pore bottom contributes to pore widening or narrowing, thereby causing the nanotube geometry to constantly evolve. A change in shape causes an alteration of band gap energy due to the stress induced processes. Raman intensities of irregular shaped TNA are stronger compared to straight or vertically grown TNA. The analyses of relative absorbance data indicate that the peak of the absorbance zone due to tapering of TNA is 90 nm away from the absorbance peak due to the tilt of TNA. The absorbance of Ag nanocrystals coated TNA shows a sharp and distinct absorbance peak at ∼320 nm due to surface plasmon resonance that matches with the peak predicted by discrete dipole approximation (DDA). An appearance of a broad absorbance (400–700 nm) peak can be explained by ‘slow photon’ effect and plasmon–exciton interaction. Such a slow photon effect is advantageous for improved photoelectrochemical performance and quantum efficiency of TiO2 based devices. The propagation of surface electromagnetic waves can be studied along the nanotube structures using the FDTD simulation tool. FDTD simulations revealed an enhanced light absorption at λ = 400 nm, corresponding to the band gap of titania. In addition, high internal fields in tilted nanotubes were observed due to waveguide modes and an enhanced field confinement at lower bend angle.References
- E. Yablonovitch, Phys. Rev. Lett., 1987, 58, 2059–2062 CrossRef CAS.
- A. Soudi, P. Dhakal and Y. Gu, Appl. Phys. Lett., 2010, 96, 253115 CrossRef PubMed.
- C.-M. Lin, Y.-C. Chang, J. Yao, C. Wang, C. Luo and S. Yin, Mater. Chem. Phys., 2012, 135, 723–727 CrossRef CAS PubMed.
- F. Shao, J. Sun, L. Gao, S. Yang and J. Luo, J. Mater. Chem., 2012, 22, 6824–6830 RSC.
- Y. Liu, B. Zhou, J. Li, X. Gan, J. Bai and W. Cai, Appl. Catal., B, 2009, 92, 326–332 CrossRef CAS PubMed.
- X. Pan, C. Chen, K. Zhu and Z. Fan, Nanotechnology, 2011, 22, 235402 CrossRef PubMed.
- H.-G. Yun, J. H. Park, B.-S. Bae and M. G. Kang, J. Mater. Chem., 2011, 21, 3558–3561 RSC.
- A. S. Nair, Z. Peining, V. J. Babu, Y. Shengyuan and S. Ramakrishna, Phys. Chem. Chem. Phys., 2011, 13, 21248 RSC.
- J. An, W. Guo and T. Ma, Small, 2012, 8, 3427–3431 CrossRef CAS PubMed.
- L. Sun, S. Zhang, Q. Wang and D. Zhao, Nanosci. Nanotechnol. Lett., 2012, 4, 471–482 CrossRef CAS PubMed.
- S. E. Skelton, M. Sergides, R. Patel, E. Karczewska, O. M. Maragó and P. H. Jones, J. Quant. Spectrosc. Radiat. Transfer, 2012, 113, 2512–2520 CrossRef CAS PubMed.
- H. S. Haddock, P. M. Shankar and R. Mutharasan, Mater. Sci. Eng., B, 2003, 97, 87–93 CrossRef.
- S. So, A. Kriesch, U. Peschel and P. Schmuki, J. Mater. Chem. A, 2015, 3, 12603–12608 CAS.
- S. L. Diedenhofen, O. T. A. Janssen, G. Grzela, E. P. A. M. Bakkers and J. Gómez Rivas, ACS Nano, 2011, 5, 2316–2323 CrossRef CAS PubMed.
- S. McCulloch and D. Uttamchandani, Meas. Sci. Technol., 1995, 6, 1157 CrossRef CAS.
- Y. Zaatar, D. Zaouk, J. Bechara, A. Khoury, C. Llinaress and J. P. Charles, Mater. Sci. Eng., B, 2000, 74, 296–298 CrossRef.
- J. Villatoro and D. Monzón-Hernández, Opt. Express, 2005, 13, 5087–5092 CrossRef CAS.
- F. J. Sánchez-Martín, P. M. Fernández-Salguero and J. M. Merino, J. Neurochem., 2011, 118, 153–162 CrossRef PubMed.
- Z. M. Hale and F. P. Payne, A tapered single-mode optical fibre as an intrinsic pH sensor, Fibre Optics Sensor Technology, IEE Colloquium, 1992, p. 8 Search PubMed.
- W.-H. Lin and Y.-Y. Li, Diamond Relat. Mater., 2012, 22, 124–127 CrossRef CAS PubMed.
- S.-A. Lee, H. S. Kang, J.-K. Park and S. Lee, Adv. Mater., 2014, 26, 7521–7528 CrossRef CAS PubMed.
- G. K. Mor, O. K. Varghese, M. Paulose, N. Mukherjee and C. A. Grimes, J. Mater. Res., 2003, 18, 2588–2593 CrossRef CAS.
- S. K. Mohapatra, M. Misra, V. K. Mahajan and K. S. Raja, Mater. Lett., 2008, 62, 1772–1774 CrossRef CAS PubMed.
- M. S. Fakir, A. Supangat and K. Sulaiman, J. Nanomater., 2014, 2014, 7 Search PubMed.
- X. Zhang, Y. Liu, S.-T. Lee, S. Yang and Z. Kang, Energy Environ. Sci., 2014, 7, 1409–1419 CAS.
- S. Wang, K. Qian, X. Bi and W. Huang, J. Phys. Chem. C, 2009, 113, 6505–6510 CAS.
- Z. Li, A. Friedrich and A. Taubert, J. Mater. Chem., 2008, 18, 1008–1014 RSC.
- L. Sun, S. Zhang and Q. Wang, J. Nanosci. Nanotechnol., 2014, 14, 2050–2064 CrossRef CAS PubMed.
- Y. Yao, C.-Y. Cheung, J. Bao and M. Skyllas-Kazacos, Light Metals 2015, Orlando, FL, 2015 Search PubMed.
- L. Sun, S. Zhang, X. W. Sun and X. He, J. Electroanal. Chem., 2009, 637, 6–12 CrossRef CAS PubMed.
- L. Sun, S. Zhang, X. Wang, X. W. Sun, D. Y. Ong, X. Wang and D. Zhao, ChemPhysChem, 2011, 12, 3634–3641 CrossRef CAS PubMed.
- P. K. Sarswat, Y. R. Smith, M. L. Free and M. Misra, ECS J. Solid State Sci. Technol., 2015, 4, Q83–Q91 CrossRef CAS PubMed.
- R. Rao, D. Liptak, T. Cherukuri, B. I. Yakobson and B. Maruyama, Nat. Mater., 2012, 11, 213–216 CrossRef CAS PubMed.
- X. Duan, H. Son, B. Gao, J. Zhang, T. Wu, G. G. Samsonidze, M. S. Dresselhaus, Z. Liu and J. Kong, Nano Lett., 2007, 7, 2116–2121 CrossRef CAS PubMed.
- Z. Jun, Y. Jing-Xin, W. Yan-Ju, C. Xiang-Rong and J. Fu-Qian, Chin. Phys. B, 2008, 17, 2216 CrossRef.
- J. P. Lu, Phys. Rev. Lett., 1997, 79, 1297–1300 CrossRef CAS.
- W.-J. Yin, S. Chen, J.-H. Yang, X.-G. Gong, Y. Yan and S.-H. Wei, Appl. Phys. Lett., 2010, 96, 221901 CrossRef PubMed.
- K. Nishidate and M. Hasegawa, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 195403 CrossRef.
- E. Khon, A. Mereshchenko, A. N. Tarnovsky, K. Acharya, A. Klinkova, N. N. Hewa-Kasakarage, I. Nemitz and M. Zamkov, Nano Lett., 2011, 11, 1792–1799 CrossRef CAS PubMed.
- M. Torrell, R. Kabir, L. Cunha, M. I. Vasilevskiy, F. Vaz, A. Cavaleiro, E. Alves and N. P. Barradas, J. Appl. Phys., 2011, 109, 074310 CrossRef PubMed.
- M. Achermann, J. Phys. Chem. Lett., 2010, 1, 2837–2843 CrossRef CAS.
- A. Mohammadpour and K. Shankar, J. Mater. Chem., 2010, 20, 8474–8477 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: XPS results, band gap information and related details. See DOI: 10.1039/c5ra12191d |
| This journal is © The Royal Society of Chemistry 2015 |