Molybdenum oxide/γ-alumina: an efficient solid acid catalyst for the synthesis of nopol by Prins reaction
Abstract
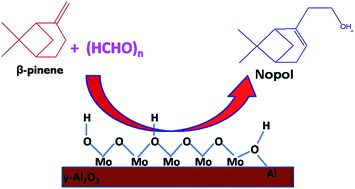
Prins condensation of β-pinene with paraformaldehyde was carried out over MoOx/γ-Al2O3 catalyst in liquid phase. MoOx/γ-Al2O3 catalysts with different loadings were synthesized by impregnation method and characterized by XRD, N2 sorption, NH3-TPD, UV-DRS, TEM and SEM. The surface area, TEM and UV-DRS measurements reveal complete coverage of alumina support by MoOx was achieved between 9 and 11 wt% loading in which the molybdenum is present as octahedral and tetrahedral species in the form of a monolayer domain and small clusters of MoOx. The effect of MoOx loading and calcination temperature of the catalysts on total acidity and catalytic activity in the Prins reaction were correlated. The total surface acid density of MoOx/γ-Al2O3 increased with an increase in MoOx loading until 11 wt% and further loading decreased the acidity. Among the different supports, ZrO2, TiO2, SiO2 and Nb2O5, γ-Al2O3 exhibited the best performance. The influence of solvent on the Prins reaction in terms of dielectric constant, acceptor and donor number was investigated. Solvents with both AN and DN in the range of 10 to 20 were suitable to facilitate the reaction. The effect of reaction temperature, catalyst amount, reactant mole ratio and reusability of the catalyst for the Prins reaction were investigated. The 11 wt% MoOx loaded on γ-Al2O3 showed the best performance with 96% β-pinene conversion with 86% nopol selectivity.


 Please wait while we load your content...
Please wait while we load your content...