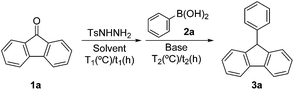
A simple and efficient synthesis of 9-arylfluorenes via metal-free reductive coupling of arylboronic acids and N-tosylhydrazones in situ†
Xu Shen,
Ningning Gu,
Ping Liu*,
Xiaowei Ma,
Jianwei Xie,
Yan Liu*,
Lin He and
Bin Dai
School of Chemistry and Chemical Engineering/Key Laboratory for Green Processing of Chemical Engineering of Xinjiang Bingtuan, Shihezi University, Shihezi 832003, China. E-mail: liuping1979112@aliyun.com; liuyan1979810@aliyun.com; Fax: +86 0993 2057270; Tel: +86 0993 2057213
First published on 21st July 2015
Abstract
A general, yet efficient synthesis method of 9-arylfluorenes via metal-free reductive coupling of N-tosylhydrazones and arylboronic acids has been developed. This methodology is realized by a one-pot protocol in two steps involving the preparation of N-tosylhydrazones by reacting tosylhydrazide with 9-fluorenone derivatives, followed by the reductive coupling of arylboronic acid in the presence of potassium carbonate to afford various 9-arylfluorenes analogues in moderate to excellent yields. Importantly, the catalytic system presented here enables the use of easily accessible starting materials and can be employed on a wide variety of substrates with good functional group tolerance. This protocol could also be particularly useful for the synthesis of 9-fluorenyl-substituted carbazolyl compounds.
Introduction
Fluorene proves to be an important structural scaffold and can be found in a variety of applications involving advanced materials, mostly due to its unique electronic and photonic properties.1 Notably, 9-arylfluorenes have attracted considerable attention as compounds with promising properties to find use in, e.g., blue fluorescent organic light emitting materials, thin film transistors, photovoltaic cells, etc.2 As a consequence, the development of new synthetic methods for the preparation of 9-arylfluorenes has been the main focus of related fields in recent years. In general, the reaction between 9-fluorenone and organomagnesium or organolithium compounds yields the corresponding carbinols, which can be transformed into 9-arylfluorenes by treatment with Et2O·BF3/Et3SiH or TsOH (cf. Method 1).3 Alternatively, a catalytic system consisting of phenyl methyl sulfoxide (PMSO) and the polyoxomolybdate [PMo12O40]3−, triphenylmethane provides 9-phenylfluorene via a triphenylmethane cation (cf. Method 2).4 Recently, a novel strategy for the preparation of 9-arylfluorenes via intramolecular tandem reactions of 2-arylbenzaldehydes with arenes catalyzed by CF3SO3H or a combination of bimetallic “Pd–Sn” and AgPF6 system has been reported (cf. Method 3).5 Additionally, Friedel–Crafts cyclization reactions of biaryl alcohols or acetates catalyzed by a Brønsted or Lewis acid, such as HCl/HOAc or BF3·Et2O, have also been shown to furnish 9-arylfluorenes (cf. Method 4).6 Moreover, 9-arylfluorenes can be obtained from 9-bromofluorene through p-toluenesulfonic acid catalyzing the intermolecular coupling reaction (cf. Method 5),7 and the zinc-mediated radical reaction (cf. Method 6).8 Although the methods listed above prove to be effective for the synthesis of 9-arylfluorenes, certain disadvantages particularly involving a narrow substrate scope and poor functional group tolerance need to be addressed. The 9-arylfluorene scaffold is usually constructed via multi-step reactions that sometimes require the use of a strongly acidic medium or a stoichiometric amount of a Lewis acid. However, it is worth noting that a transition metal catalyzed coupling reaction has been developed for the direct synthesis of 9-arylfluorenes. For example, Chandrasekhar et al. developed a catalytic protocol for the synthesis of 9-arylfluorenes via palladium-catalyzed Suzuki–Miyaura coupling of 9-bromofluorene with arylboronic acids (cf. Method 7).9 Wu et al. reported a catalytic system consisting of palladium(II) acetate and tricyclohexylphosphine with the reaction of fluorene with haloarenes to provide 9-arylfluorenes in good to excellent yields (cf. Method 8).10 The synthetic protocol proposed here will not only mitigate the mentioned disadvantages of Methods 1–6, but will also efficiently reduce the number of reaction steps needed to carry out the synthesis.N-tosylhydrazones prove to be highly versatile synthetic intermediates that have attracted considerable interest in a variety of research fields in recent years.11 In 2007, Valdés12 and coworkers from Wang's group13 developed a series of transformation reactions with tosylhydrazones. To the best of our knowledge, as early as 2009, Valdés et al. have developed a new metal-free C–C bond formation reaction between N-tosylhydrazones and boronic acid derivatives that proved to be suitable for the preparation of biarylmethane structures. This process can be carried out without the need for a metal catalyst and adapting extremely simple reaction conditions exhibiting remarkably versatile applications.12b Inspired by this work and in contrast to the methods mentioned above, requiring a multi-step process for the preparation of 9-arylfluorenes and the use a strongly acidic medium, a Lewis acid, or a palladium catalyst promote these catalytic reactions, we herein report a new method that employs 9-fluorenone derivatives as a simple and readily available starting material for the one-pot, two-step synthesis of 9-arylfluorenes through the metal-free reductive coupling of N-tosylhydrazones and arylboronic acids (cf. Method 9) (Scheme 1).
Results and discussion
Optimization of the reaction conditions
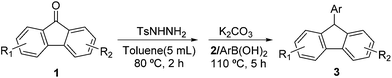
Initially, 9-fluorenone (0.5 mmol) and phenylboronic acid (0.75 mmol) were chosen as model substrates for the reductive coupling in the presence of tosylhydrazide (1.5 equivalents) and potassium carbonate (K2CO3, 2 equivalents). Tosylhydrazones prove to be easily accessible by reacting tosylhydrazide with a corresponding ketone. Therefore, we focussed our studies on the reductive coupling in an effort to answer the question if this reaction could be carried out in a one-pot fashion directly from 9-fluorenone and without the need to isolate the intermediate, tosylhydrazone. Indeed, this process proves to be feasible and can be carried out by simply heating 9-fluorenone with tosylhydrazide for 2 hours at 80 °C prior to the addition of phenylboronic acid. Heating was then continued for another 5 hours at 110 °C and the product of this reductive coupling, 9-phenyl-9H-fluorene, was obtained in 34% yield using 1,4-dioxane as a solvent. Furthermore, the intermediate tosylhydrazone did not have to be isolated (cf. Table 1, entry 2). A short list of solvents with similar boiling points as 1,4-dioxane have been screened for applicability in this reaction. Notably, significantly higher yields have been obtained using nonpolar solvent such as toluene; a lower yield has been observed using dimethylformamide (DMF, cf. Table 1, entries 2 and 3) as solvent. The use of tetrahydrofuran (THF) was also investigated, but the high volatility of the solvent forced us to lower the reaction temperature to 65 °C, resulting in a particularly low yield obtained (cf. Table 1, entry 4). The screening of different bases revealed that K2CO3 proves to be the most appropriate base for delivering the desired product in 89% yield (cf. Table 1, entry 11), while other bases such as K3PO4, Na2CO3, NaOH, KOH, and Cs2CO3 afforded significantly lower yields (31–70%, cf. Table 1, entries 5–9). Meanwhile, the base loading was found to be another crucial parameter with the product yield increasing from 80 to 89% as the base loading increases from 1.5 equivalents to 3.0 equivalents (cf. Table 1, entries 2, 10 and 11), respectively. In summary, the combination of 9-fluorenone (0.5 mmol), tosylhydrazide (1.5 equiv.), phenylboronic acid (0.75 mmol), K2CO3 (2 equivalents) at T1 = 80 °C (t1 = 2 hours) and T2 = 110 °C (t2 = 5 hours) in toluene (5 mL) were found to be the most suitable reaction conditions.| Entry | Base | Solvent | Yield (%)b |
|---|---|---|---|
| a Reaction conditions: reaction conditions: 0.50 mmol 9-fluorenone, tosylhydrazide (1.5 equiv.), 0.75 mmol phenylboronic acid, 5 mL toluene, T1 = 80 °C/t1 = 2 h, T2 = 110 °C/t2 = 5 h.b GC-MS yield.c T1 = T2 = 65 °C, yield of GC-MS. | |||
| 1 | K2CO3 (1.5 equiv.) | 1,4-Dioxane | 34 |
| 2 | K2CO3 (1.5 equiv.) | Toluene | 80 |
| 3 | K2CO3 (1.5 equiv.) | DMF | Trace |
| 4c | K2CO3 (1.5 equiv.) | THF | 41 |
| 5 | K3PO4 (1.5 equiv.) | Toluene | 67 |
| 6 | Na2CO3 (1.5 equiv.) | Toluene | 31 |
| 7 | NaOH (1.5 equiv.) | Toluene | 46 |
| 8 | KOH (1.5 equiv.) | Toluene | 48 |
| 9 | Cs2CO3 (1.5 equiv.) | Toluene | 70 |
| 10 | K2CO3 (2 equiv.) | Toluene | 87 |
| 11 | K2CO3 (3 equiv.) | Toluene | 89 |
Scope and limitations of substrates
Upon optimizing the reaction conditions, we further investigated the substrate scope of this one pot, two-step reductive coupling reaction. As shown in Table 2, the 4-substituted arylboronic acid substrates bearing electron-withdrawing or electron-donating groups constantly afford the desired products 3b–3g in good to excellent yields (78–92%). Moreover, (3,4,5-trifluorophenyl)boronic acid and m-tolylboronic acid were found to produce the desired coupling products 3h and 3i in 79% and 88% yields, respectively. Naphthalene-2-ylboronic acid has also been used as a coupling partner to provide the product 3j in 77% yield. Furthermore, the reductive coupling reactions involving ortho-substituted arylboronic acids with 9-fluorenone have been investigated. Both the electronic properties and the steric hindrance of the substrates seem to influence the coupling reaction. For example, the coupling reaction of o-tolylboronic acid with 9-fluorenone to afford the product 3k proceeds in 75% yield.| a Reaction conditions: 0.50 mmol substituted 9-fluorenone, 0.75 mmol phenylboronic acid, 1.5 equiv. base, 5 mL toluene, T1 = 80 °C/t1 = 2 h, T2 = 110 °C/t2 = 5 h. The yields of isolated products are given. |
|---|
 |
However, (2,3-difluorophenyl)boronic acid and (2,4-difluorophenyl)boronic acid as substrates result in the formation of the corresponding coupling products 3l and 3m in low yields of 35% and 37%, respectively. Similarly, while thiophen-3-ylboronic acid shows good reactivity towards conversion into the corresponding product 3n in 85% yield, the use of furan-2-ylboronic acid as the substrate results in the formation of product 3o only in 29% yield. Noteworthy, the reaction of butylboronic acid with 9-fluorenone has also been attempted using standard reaction conditions. The desired product 3p was obtained in 92% yield. To further investigate the range of substrates that can be used in this process, a variety of substituted 9-fluorenone derivatives have been studied. The results show that the coupling reactions involving substituted 9-fluorenone and (4-methoxyphenyl)boronic acid work to our satisfaction and afford the corresponding products 3q–3u in yields ranging from 62% to 93%. The synthesis of substituted carbazolyl compounds has attracted considerable interest due to the importance of this compound in numerous photo devices, electroluminescent devices and photorefractive materials. However, to the best of our knowledge no reports on the synthesis of 9-fluorenyl-substituted carbazolyl compounds via metal free reductive coupling reactions have been published to date. Therefore, this method provided further incentive to be applied to the synthesis of 9-fluorenyl-substituted carbazolyl compounds. The reaction of 9-fluorenone with (3-(9H-carbazol-9-yl)phenyl)boronic acid, (9-phenyl-9H-carbazol-3-yl)boronic acid and (4-(9H-carbazol-9-yl)phenyl)boronic acid was carried out efficiently and the desired products 3v–3x have been isolated in high yields ranging from 62 to 91%. Furthermore, (4-(diphenylamino)phenyl)boronic acid can also react with 9-fluorenone to afford the functionalized coupling product 3y in 65% yield.
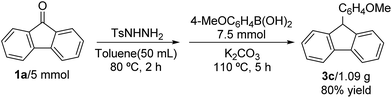
A gram-scale synthesis was performed to verify the practical application using this synthesis system. Fortunately, the reaction was performed using 5 mmol of 9-fluorenone and 7.5 mmol (4-methoxyphenyl)boronic acid, and proceeded in 80% yield leading to 1.08 g of the desired product 3c (Scheme 2).
Conclusions
To summarize, a simple and efficient method for the synthesis of 9-arylfluorenes via metal-free reductive coupling of arylboronic acids with N-tosylhydrazones in situ has been developed, affording the corresponding target molecules in moderate to excellent yields. Moreover, isolation of the desired tosylhydrazones is not required and the reaction can be carried out in one pot and in two steps, directly from the 9-fluorenone derivatives. Potential substrates for this reaction are diverse, exhibiting a superior functional-group tolerance. Furthermore, no sophisticated experimental setups, e.g. involving inert gasses or dry solvents, are required. Notably, this protocol also proves suitable for the synthesis of 9-fluorenyl-substituted carbazolyl compounds. Further studies to investigate employing this system to other related reactions are currently underway in our laboratory.Experimental
Materials and instruments
Chemicals were obtained commercially and used as received. NMR spectra were recorded on a Bruker DPX–400 spectrometer using TMS as the internal standard. EI–Mass spectrum was measured on a LC/Q–TOF MS (Micromass, England) All products were isolated by short chromatography on a silica gel (200–300 mesh) column using petroleum ether (60–90 °C), unless otherwise noted. 9-Fluorenone and its derivatives, and arylboronic acids were of analytical grade quality, purchased from Adamas-beta Pharmaceuticals, Inc.General procedure for the one-pot two-steps reductive coupling of N-tosylhydrazones and arylboronic acids
A solution of the 9-fluorenone derivatives 1 (0.5 mmol) and tosylhydrazide (0.75 mmol) in 5 mL of toluene was stirred at 80 °C for 2 h in a reaction tube. Potassium carbonate (1.0 mmol) and the appropriate boronic acid 2 (0.75 mmol) were added to the reaction mixture. The system was refluxed at 110 °C for 5 h with stirring. When the reaction was complete, the crude mixture was allowed to reach room temperature. Dichloromethane and a saturated solution of NaHCO3 were added and the layers were separated. The aqueous phase was extracted three times with dichloromethane. The combined organic layers were washed with a saturated solution of NaHCO3, one portion of brine and then dried over Na2SO4 and filtered. The solvent was removed under reduced pressure. The products were purified by chromatography on silica gel.9-(4-Propylphenyl)-9H-fluorene [3d]
Mp. 88–89 °C. 1H NMR (400 MHz, CDCl3) δ 7.79 (dd, J = 7.6, 0.8 Hz, 2H), 7.40–7.31 (m, 4H), 7.29–7.22 (m, 2H), 7.03 (ddd, J = 9.8, 7.2, 1.9 Hz, 4H), 5.02 (s, 1H), 2.57–2.51 (m, 2H), 1.61 (ddd, J = 9.1, 7.5, 4.1 Hz, 2H), 0.96–0.90 (m, 3H). 13C NMR (101 MHz, CDCl3) δ 148.09 (s), 141.20 (s), 140.98 (s), 138.68 (s), 128.76 (s), 128.13 (s), 127.25 (dd, J = 3.0, 2.1 Hz), 125.35 (s), 119.83 (s), 77.36 (s), 77.04 (s), 76.72 (s), 54.13 (s), 37.74 (s), 24.52 (s), 13.94 (s). HRMS (EI): m/z calcd for C22H24 [M]+ 284.1565, found 284.1563. IR (KBr, cm−1): 2953, 2928, 2826, 1510, 1448, 738.9-(4-Pentylphenyl)-9H-fluorene [3e]
Mp.74–75 °C. 1H NMR (400 MHz, CDCl3) δ 7.79 (d, J = 7.6 Hz, 2H), 7.39–7.31 (m, 4H), 7.25 (td, J = 7.2, 1.1 Hz, 3H), 7.07 (d, J = 8.2 Hz, 2H), 7.00–6.97 (m, 2H), 5.02 (s, 1H), 2.58–2.52 (m, 2H), 1.63–1.53 (m, 3H), 1.34–1.24 (m, 5H), 0.88 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 148.07 (s), 141.45 (s), 140.97 (s), 138.61 (s), 128.68 (s), 128.13 (s), 127.23 (d, J = 4.2 Hz), 125.34 (s), 119.82 (s), 77.34 (s), 77.02 (s), 76.71 (s), 54.11 (s), 35.61 (s), 31.61 (s), 31.14 (s), 22.56 (s), 14.05 (s). HRMS (EI): m/z calcd for C24H24 [M]+ 312.1878, found 312.1873. IR (KBr, cm−1): 3048, 2951, 2876, 1510, 1449, 739.9-(3,4,5-Trifluorophenyl)-9H-fluorene [3h]
Mp. 115.7–116.8 °C. 1H NMR (400 MHz, CDCl3) δ 7.79 (dd, J = 6.9, 0.7 Hz, 2H), 7.43–7.38 (m, 2H), 7.30–7.27 (m, 4H), 6.70 (dd, J = 8.5, 6.5 Hz, 2H), 4.95 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 146.28 (s), 140.94 (s), 127.96 (s), 127.62 (s), 125.14 (s), 120.18 (s), 112.20 (dd, J = 15.6, 5.8 Hz), 86.82 (s), 77.35 (s), 77.03 (s), 76.71 (s), 53.39 (s). 19F NMR (376 MHz, CDCl3) δ −134.06 (d, J = 20.7 Hz), −162.77 (t, J = 20.6 Hz). HRMS (EI): m/z calcd for C19H11F3 [M]+ 296.0813, found 296.0810. IR (KBr, cm−1): 3048, 2951, 2876, 1510, 1449, 739.9-(2,3-Difluorophenyl)-9H-fluorene [3l]
Mp. 100–101 °C. 1H NMR (400 MHz, CDCl3) δ 7.80 (d, J = 7.6 Hz, 2H), 7.43–7.34 (m, 4H), 7.28 (td, J = 7.4, 1.1 Hz, 2H), 7.07–6.98 (m, 1H), 6.84 (tdd, J = 8.1, 5.0, 1.7 Hz, 1H), 6.42 (dd, J = 7.7, 6.3 Hz, 1H), 5.49 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 146.20 (d, J = 1.1 Hz), 141.16 (s), 131.22 (d, J = 11.9 Hz), 127.57 (d, J = 17.4 Hz), 125.19 (d, J = 1.2 Hz), 124.15 (dd, J = 7.0, 4.7 Hz), 123.80 (s), 120.07 (s), 115.58 (d, J = 17.0 Hz), 77.34 (s), 77.02 (s), 76.71 (s), 29.72 (s). 19F NMR (376 MHz, CDCl3) δ −137.94 to −138.01 (m), −143.90 (d, J = 19.9 Hz). HRMS (EI): m/z calcd for C19H12F2 [M]+ 278.0907, found 278.0909. IR (KBr, cm−1): 1485, 1446, 1280, 945, 800, 779, 742.9-(2,4-Difluorophenyl)-9H-fluorene [3m]
Mp. 94.6–95.3 °C. 1H NMR (400 MHz, CDCl3) δ 7.80 (d, J = 7.6 Hz, 2H), 7.40 (t, J = 7.4 Hz, 2H), 7.34 (d, J = 7.4 Hz, 2H), 7.28 (dd, J = 7.4, 1.0 Hz, 2H), 6.94–6.87 (m, 1H), 6.70–6.59 (m, 2H), 5.42 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 146.55 (s), 141.11 (s), 127.51 (d, J = 12.4 Hz), 125.13 (d, J = 1.2 Hz), 120.05 (s), 111.74–111.25 (m), 104.12 (s), 103.87 (s), 77.34 (s), 77.02 (s), 76.70 (s). 19F NMR (376 MHz, CDCl3) δ −112.22 (dd, J = 6.8, 1.1 Hz), −114.77 (s). HRMS (EI): m/z calcd for C19H12F2 [M]+ 278.0907, found 278.0909. IR (KBr, cm−1): 1620, 1604, 1500, 1447, 1267, 964, 852, 739.2-Bromo-9-(4-methoxyphenyl)-9H-fluorene [3q]
Mp. 107–108 °C. 1H NMR (400 MHz, CDCl3) δ 7.75 (d, J = 7.6 Hz, 1H), 7.64 (d, J = 8.1 Hz, 1H), 7.49 (ddd, J = 8.1, 1.8, 0.7 Hz, 1H), 7.44–7.32 (m, 2H), 7.32–7.23 (m, 2H), 7.02–6.94 (m, 2H), 6.86–6.79 (m, 2H), 4.97 (s, 1H), 3.78 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 158.74 (s), 150.23 (s), 147.90 (s), 139.88 (d, J = 2.1 Hz), 132.57 (s), 130.43 (s), 129.29 (s), 128.54 (s), 127.73 (s), 127.47 (s), 125.35 (s), 121.11 (d, J = 14.6 Hz), 119.92 (s), 114.26 (s), 55.27 (s), 53.61 (s). HRMS (EI): m/z calcd for C20H15BrO [M]+ 350.0306, found 350.0305. IR (KBr, cm−1): 1508, 1460, 1258, 1176, 742.2,7-Dibromo-9-(4-methoxyphenyl)-9H-fluorene [3r]
Mp. 153.5–155.5 °C. 1H NMR (400 MHz, CDCl3) δ 7.57 (d, J = 8.1 Hz, 2H), 7.46 (ddd, J = 8.1, 1.8, 0.7 Hz, 2H), 7.40–7.36 (m, 2H), 6.92 (s, 2H), 6.81 (s, 2H), 4.92 (s, 1H), 3.76 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 158.95 (s), 149.88 (s), 138.85 (s), 131.60 (s), 130.69 (s), 129.29 (d, J = 1.0 Hz), 128.61 (s), 121.52 (s), 121.25 (s), 114.42 (s), 77.36 (s), 77.04 (s), 76.73 (s), 55.30 (s), 53.50 (s). HRMS (EI): m/z calcd for C20H14Br2O [M]+ 429.9391, found 429.9387. IR (KBr, cm−1): 1510, 1456, 1258, 964, 806.9-(4-Methoxyphenyl)-2-nitro-9H-fluorene [3s]
Mp. 149.8–151.1 °C. 1H NMR (400 MHz, CDCl3) δ 8.30 (d, J = 15.8 Hz, 1H), 8.15 (s, 1H), 7.88 (d, J = 8.4 Hz, 2H), 7.39 (dd, J = 5.8, 1.0 Hz, 3H), 7.00 (d, J = 8.6 Hz, 2H), 6.84 (d, J = 8.8 Hz, 2H), 5.09 (s, 1H), 3.79 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 158.98 (s), 149.76 (s), 149.22 (s), 147.22 (s), 138.63 (s), 131.39 (s), 129.33 (d, J = 19.9 Hz), 127.90 (s), 125.70 (s), 123.57 (s), 121.26 (s), 120.77 (s), 119.97 (s), 114.46 (s), 77.33 (s), 77.01 (s), 76.69 (s), 55.29 (s), 53.72 (s). HRMS (EI): m/z calcd for C20H15NO3 [M − H]+ 316.0968, found 316.0973. IR (KBr, cm−1): 1510, 1333, 1258, 1030, 752.2-Bromo-9-(4-methoxyphenyl)-7-nitro-9H-fluorene [3t]
Mp. 191.5–192.8 °C. 1H NMR (400 MHz, CDCl3) δ 8.32 (dd, J = 8.2, 1.8 Hz, 1H), 8.16 (s, 1H), 7.88 (d, J = 8.4 Hz, 1H), 7.76 (d, J = 8.2 Hz, 1H), 7.64–7.52 (m, 2H), 7.03–6.98 (m, 2H), 6.90–6.85 (m, 2H), 5.10 (s, 1H), 3.82 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 159.20 (s), 151.59 (s), 148.94 (s), 147.48 (s), 146.10 (s), 137.55 (s), 131.23 (s), 130.45 (s), 129.24 (s), 129.02 (s), 123.70 (d, J = 8.0 Hz), 122.50 (s), 120.85 (s), 120.12 (s), 114.63 (s), 77.33 (s), 77.01 (s), 76.69 (s), 55.32 (s), 53.64 (s). HRMS (EI): m/z calcd for C20H14BrNO3 [M − H]+ 394.0073, found 394.0080. IR (KBr, cm−1): 1600, 1510, 1337, 1250, 1028, 818, 737.9-(4-Methoxyphenyl)-9H-fluoren-2-amine [3u]
Mp. 161.4–162 °C. 1H NMR (400 MHz, CDCl3) δ 7.63 (d, J = 7.5 Hz, 1H), 7.56 (d, J = 8.1 Hz, 1H), 7.30 (t, J = 7.5 Hz, 1H), 7.22 (d, J = 7.4 Hz, 1H), 7.14 (dd, J = 7.4, 1.0 Hz, 1H), 7.03–6.99 (m, 2H), 6.83–6.78 (m, 2H), 6.70 (dd, J = 8.1, 2.2 Hz, 1H), 6.63 (s, 1H), 4.89 (s, 1H), 3.77 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 158.45 (s), 150.13 (s), 147.34 (s), 141.36 (s), 133.98 (s), 129.35 (s), 127.13 (s), 125.63 (s), 125.00 (s), 120.68 (s), 118.54 (s), 114.42 (s), 114.05 (s), 112.01 (s), 77.34 (s), 77.02 (s), 76.71 (s), 55.24 (s), 53.54 (s). HRMS (EI): m/z calcd for C20H17NO [M]+ 287.1305, found 287.1311. IR (KBr, cm−1): 3564, 3412, 1609, 1510, 1456, 1252, 1179, 1034, 825, 741.9-(3-(9H-Fluoren-9-yl)phenyl)-9H-carbazole [3v]
Mp. 200.3–201.1 °C. 1H NMR (400 MHz, CDCl3) δ 8.27–8.00 (m, 2H), 7.79 (dd, J = 7.5, 0.8 Hz, 2H), 7.52–7.47 (m, 1H), 7.46–7.23 (m, 14H), 7.19 (s, 1H), 5.15 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 147.39 (s), 143.82 (s), 141.08 (s), 140.69 (s), 137.96 (s), 130.13 (s), 127.53 (d, J = 15.0 Hz), 127.24 (s), 126.88 (s), 125.89 (s), 125.29 (d, J = 2.2 Hz), 123.35 (s), 120.38–119.80 (m), 109.76 (s), 77.36 (s), 77.04 (s), 76.72 (s), 54.17 (s). HRMS (EI): m/z calcd for C31H21N [M]+ 407.1674, found 407.1672. IR (KBr, cm−1): 3045, 1599, 1449, 1334, 1226, 1179, 1034, 825, 741.3-(9H-Fluoren-9-yl)-9-phenyl-9H-carbazole [3w]
Mp. 101.1–101.9 °C. 1H NMR (400 MHz, CDCl3) δ 8.05 (d, J = 7.8 Hz, 1H), 7.93 (d, J = 1.6 Hz, 1H), 7.84 (d, J = 7.6 Hz, 2H), 7.59–7.51 (m, 4H), 7.39 (ddd, J = 11.5, 9.8, 7.3 Hz, 7H), 7.29–7.25 (m, 3H), 7.24 (d, J = 1.2 Hz, 1H), 7.03 (d, J = 1.7 Hz, 1H), 5.24 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 148.72 (s), 141.04 (d, J = 19.5 Hz), 137.73 (s), 133.02 (s), 129.83 (s), 127.25 (dd, J = 22.9, 11.6 Hz), 126.33 (s), 125.92 (s), 125.47 (s), 123.61 (s), 123.14 (s), 120.37 (s), 120.07 (s), 119.86 (d, J = 2.3 Hz), 110.08 (s), 109.77 (s), 77.35 (s), 77.03 (s), 76.71 (s), 54.64 (s). HRMS (EI): m/z calcd for C31H21N [M]+ 407.1674, found 407.1672. IR (KBr, cm−1): 3267, 1595, 1501, 1448, 1323, 1234, 806, 735.9-(4-(9H-Fluoren-9-yl)phenyl)-9H-carbazole [3x]
Mp. 243.5–244.7 °C. 1H NMR (400 MHz, CDCl3) δ 8.13 (dd, J = 7.7, 0.8 Hz, 2H), 7.84 (dd, J = 7.2, 1.4 Hz, 2H), 7.48–7.38 (m, 10H), 7.37–7.25 (m, 6H), 5.18 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 147.53 (s), 141.19–140.76 (m), 136.35 (s), 129.70 (s), 127.82–127.10 (m), 125.87 (s), 125.43 (s), 123.32 (s), 120.28 (s), 119.96 (d, J = 20.0 Hz), 109.85 (s), 77.35 (s), 77.04 (s), 76.72 (s), 54.07 (s). HRMS (EI): m/z calcd for C31H21N [M]+ 407.1674, found 407.1672. IR (KBr, cm−1): 3059, 1599, 1510, 1450, 1223, 745.4-(9H-Fluoren-9-yl)-N,N-diphenylaniline [3y]
Mp. 174.2–175.1 °C. 1H NMR (400 MHz, CDCl3) δ 7.79 (s, 2H), 7.38 (d, J = 8.3 Hz, 4H), 7.29 (s, 2H), 7.19 (s, 4H), 7.05 (ddd, J = 4.4, 3.4, 1.8 Hz, 4H), 6.95 (s, 6H), 5.00 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 147.85 (d, J = 10.8 Hz), 140.96 (s), 129.09 (d, J = 15.2 Hz), 127.26 (d, J = 2.8 Hz), 125.36 (s), 124.16 (d, J = 7.2 Hz), 122.61 (s), 119.87 (s), 77.34 (s), 77.02 (s), 76.70 (s), 53.84 (s). HRMS (EI): m/z calcd for C31H23N [M]+ 409.1830, found 409.1823. IR (KBr, cm−1): 3036, 1585, 1495, 1448, 1328, 1273, 738, 649.Acknowledgements
We gratefully acknowledge financial support of this work by the National Natural Science Foundation of China (no. 21103114, no. 21463022), and Shihezi University Training Programme for Distinguished Youth Scholars (no. 2014ZRKXJQ05), Key Scientific and Technological Project of Shihezi University (gxjs2013-zdgg0201), and Start-Up Foundation for Young Scientists of Shihezi University (RCZX201408).Notes and references
- (a) H. Reisch, U. Wiester, U. Scherf and N. Tuytuylkov, Macromolecules, 1996, 29, 8204 CrossRef CAS; (b) C. Xia and R. C. Advincula, Macromolecules, 2001, 34, 6922 CrossRef CAS; (c) M. Ranger, D. Rondeau and M. Leclerc, Macromolecules, 2002, 35, 2426 CrossRef; (d) S. Merlet, M. Birau and Z. Y. Wang, Org. Lett., 2002, 4, 2157 CrossRef CAS PubMed; (e) U. Scherf and E. J. W. List, Adv. Mater., 2002, 14, 477 CrossRef CAS; (f) D. Katsis, Y. H. Geng, J. J. Ou, S. W. Culligan, A. Trajkovska, S. H.Chen and L. J. Rothberg, Chem. Mater., 2002, 14, 1332 CrossRef CAS; (g) X. Cao, W. Zhang, J. Wang, X. Zhou, H. Lu and J. J. Pei, J. Am. Chem. Soc., 2003, 125, 12430 CrossRef CAS PubMed; (h) T. Hadizad, J. Zhang, Z. Y. Wang, T. C. Gorjanc and C. Py, Org. Lett., 2005, 7, 795 CrossRef CAS PubMed; (i) F. Jaramillo-Isaza and M. L. J. Turner, Mater. Chem., 2006, 16, 83 RSC; (j) L. Xie, T. Fu, X. Hou, C. Tang, Y. Hua, R. Wang, Q. Fan, B. Peng, W. Wei and W. Huang, Tetrahedron Lett., 2006, 47, 6421 CrossRef CAS PubMed; (k) K. Wong, L. Chi, S. L. Huang, Y. iao, Y. Liu and Y. Wang, Org. Lett., 2006, 8, 5029 CrossRef CAS PubMed; (l) K. Wong, T. Hwu, A. Balaiah, T. Chao, F. Fang, C. Lee and Y. Peng, Org. Lett., 2006, 8, 1415 CrossRef CAS PubMed.
- (a) K.-T. Wong, T.-Y. Hwu, A. Balaiah, T.-C. Chao, F.-C. Fang, C.-T. Lee and Y.-C. Peng, Org. Lett., 2006, 8, 1415 CrossRef CAS PubMed; (b) F. C. Fang, C. C. Chu, C. H. Huang, G. Raffy, A. Del Guerzo, K. T. Wong and D. M. Bassani, Chem. Commun., 2008, 6369 RSC; (c) Z. Peng, S. Tao and X. Zhang, J. Phys. Chem. C, 2008, 113, 2165 CrossRef; (d) L. Rong, Q. Liu, Y. Shi and J. Tang, Chem. Commun., 2011, 47, 2155 RSC; (e) H.-g. Li, G. Wu, H.-Z. Chen and M. Wang, Org. Electron., 2011, 12, 70 CrossRef CAS PubMed; (f) L. H. Xie, X. Y. Hou, Y. R. Hua, C. Tang, F. Liu, Q. L. Fan and W. Huang, Org. Lett., 2006, 8, 3701 CrossRef CAS PubMed.
- (a) G. C. Vougioukalakis, M. M. Roubelakis and M. Orfanopoulos, J. Org. Chem., 2010, 75, 4124 CrossRef CAS PubMed; (b) M. Y. Teng, Y. Liu, S. L. Li, G. Huang, J. Jiang and L. Wang, RSC Adv., 2013, 3, 9016 RSC.
- A. M. Khenkin and R. Neumann, J. Am. Chem. Soc., 2002, 124, 4198 CrossRef CAS PubMed.
- (a) Q. Li, W. Xu, J. Hu, X. Chen, F. Zhang and H. Zheng, TfOH catalyzed synthesis of 9-arylfluorenes via tandem reaction under warm and efficient conditions, RSC Adv., 2014, 4, 27722 RSC; (b) D. Das, S. Pratihar and S. Roy, Org. Lett., 2012, 14, 4870 CrossRef CAS PubMed.
- (a) S. Sarkar, S. Maiti, K. Bera, S. Jalal and U. Jana, Tetrahedron Lett., 2012, 53, 5544 CrossRef CAS PubMed; (b) K. Kobiro, M. Matsura, H. Kojima and K. Nakahara, Tetrahedron, 2009, 65, 807 CrossRef CAS PubMed; (c) G. Li, E. Wang, H. Chen, H. Li, Y. Liu and P. G. Wang, Tetrahedron, 2008, 64, 9033 CrossRef CAS PubMed.
- M. P. D. Mahindaratne and K. J. Wimalasena, J. Org. Chem., 1998, 63, 2858 CrossRef CAS.
- J. Wang, W. Wan, H. Jiang, Y. Gao, X. Jiang, H. Lin, W. Zhao and J. Hao, Org. Lett., 2010, 12, 3874 CrossRef CAS PubMed.
- V. Chandrasekhar, R. S. Narayanan and P. Thilagar, Organometallics, 2009, 28, 5883 CrossRef CAS.
- J. J. Chen, S. Onogi, Y. C. Hsieh, C. C. Hsiao, S. Higashibayashi, H. Sakurai and Y. T. Wu, Adv. Synth. Catal., 2012, 354, 1551 CrossRef CAS PubMed.
- (a) V. K. Aggarwal, E. Alonso, G. Fang, M. Ferrara, G. Hynd and M. Porcelloni, Angew. Chem., 2001, 113, 1482 (Angew. Chem., Int. Ed., 2001, 40, 1433) CrossRef; (b) V. K. Aggarwal, E. Alonso, G. Hynd, K. M. Lydon, M. J. Palmer, M. Porcelloni and J. R. Studley, Angew. Chem., 2001, 113, 1479 (Angew. Chem., Int. Ed., 2001, 40, 1430) CrossRef; (c) V. K. Aggarwal, J. R. Fulton, C. G. Sheldon and J. de Vicente, J. Am. Chem. Soc., 2003, 125, 6034 CrossRef CAS PubMed; (d) K. Inamoto, T. Saito, M. Katsuno, T. Sakamoto and K. Hiroya, Org. Lett., 2007, 9, 2931 CrossRef CAS PubMed.
- (a) J. Barluenga, P. Moriel, C. Valdes and F. Aznar, Angew. Chem., 2007, 119, 5683 (Angew. Chem., Int. Ed., 2007, 46, 5587) CrossRef PubMed; (b) J. Barluenga, M. Tomás-Gamasa, F. Aznar and C. Valdés, Nat. Chem., 2009, 1, 494 CrossRef CAS PubMed; (c) J. Barluenga, M. Escribano, F. Aznar and C. Valdés, Angew. Chem., 2010, 122, 7008 (Angew. Chem., Int. Ed., 2010, 49, 6856) CrossRef PubMed; (d) J. Barluenga, L. Florentino, F. Aznar and C. Valdés, Org. Lett., 2011, 13, 510 CrossRef CAS PubMed; (e) J. Barluenga, M. Tomás-Gamasa, F. Aznar and C. Valdés, Angew. Chem., 2010, 122, 5113 (Angew. Chem., Int. Ed., 2010, 49, 4993) CrossRef PubMed; (f) J. Barluenga, N. Quinones, M. P. Cabal, F. Aznar and C. Valdes, Angew. Chem., 2011, 123, 2398 (Angew. Chem., Int. Ed., 2011, 50, 2350) CrossRef PubMed; (g) J. Barluenga and C. Valdés, Angew. Chem., 2011, 123, 7626 (Angew. Chem., Int. Ed., 2011, 50, 7486) CrossRef PubMed; (h) R. Barroso, R. A. Valencia, M. P. Cabal and C. Valdés, Org. Lett., 2014, 16, 2264 CrossRef CAS PubMed; (i) L. Florentino, F. Aznar and C. Valdés, Chemistry., 2013, 19, 10506 CrossRef CAS PubMed; (j) M. C. Pérez-Aguilar and C. Valdés, Angew. Chem., Int. Ed., 2012, 51, 5953 CrossRef PubMed; (k) J. Barluenga, M. Tomás-Gamasa and C. Valdés, Angew. Chem., Int. Ed., 2012, 51, 5950 CrossRef CAS PubMed; (l) L. Florentino, F. Aznar and C. Valdés, Org. Lett., 2012, 14, 2323 CrossRef CAS PubMed; (m) M. C. Pérez-Aguilar and C. Valdés, Angew. Chem., Int. Ed., 2013, 52, 7219 CrossRef PubMed.
- (a) Q. Xiao, J. Ma, Y. Yang, Y. Zhang and J. B. Wang, Org. Lett., 2009, 11, 4732 CrossRef CAS PubMed; (b) L. Zhou, F. Ye, Y. Zhang and J. B. Wang, J. Am. Chem. Soc., 2010, 132, 13590 CrossRef CAS PubMed; (c) Q. Xiao, Y. Xia, H. A. Li, Y. Zhang and J. B. Wang, Angew. Chem., 2011, 123, 1146 (Angew. Chem., Int. Ed., 2011, 50, 1114) CrossRef PubMed; (d) L. Zhou, Y. Shi, Q. Xiao, Y. Z. Liu, F. Ye, Y. Zhang and J. B. Wang, Org. Lett., 2011, 13, 968 CrossRef CAS PubMed; (e) L. Zhou, F. Ye, J. C. Ma, Y. Zhang and J. B. Wang, Angew. Chem., 2011, 123, 3572 (Angew. Chem., Int. Ed., 2011, 50, 3510) CrossRef PubMed; (f) X. Zhao, G. Wu, Y. Zhang and J. Wang, J. Am. Chem. Soc., 2011, 133, 3296 CrossRef CAS PubMed; (g) F. Ye, X. Ma, Q. Xiao, Y. Zhang and J. Wang, J. Am. Chem. Soc., 2012, 134, 5742 CrossRef CAS PubMed; (h) Q. Xiao, L. Ling, F. Ye, R. Tan, L. Tian, Y. Zhang, Y. Li and J. Wang, J. Org. Chem., 2013, 78, 3879 CrossRef CAS PubMed; (i) S. Xu, G. Wu, F. Ye, X. Wang, H. Li, X. Zhao, Y. Zhang and J. Wang, Angew. Chem., Int. Ed., 2015, 54, 4669 CrossRef CAS PubMed; (j) Z. Zhang, Q. Zhou, W. Yu, T. Li, G. Wu, Y. Zhang and J. Wang, Org. Lett., 2015, 17, 2474 CrossRef CAS PubMed; (k) Z. Zhang, Q. Zhou, F. Ye, Y. Xia, G. Wu, M. L. Hossain, Y. Zhang and J. Wang, Adv. Synth. Catal., 2015, 357, 2277 CrossRef CAS PubMed; (l) Z. Zhang, J. Feng, Y. Xu, S. Zhang, Y. Ye, T. Li, X. Wang, J. Chen, Y. Zhang and J. Wang, Synlett, 2015, 26, 59 CAS; (m) F. Ye, C. Wang, X. Ma, M. L. Hossain, Y. Xia, Y. Zhang and J. Wang, J. Org. Chem., 2015, 80, 647 CrossRef CAS PubMed; (n) Y. Xia, Y. Xia, Y. Zhang and J. Wang, Org. Biomol. Chem., 2014, 12, 9333 RSC; (o) M. L. Hossain, F. Ye, Z. Liu, Y. Xia, Y. Shi, L. Zhou, Y. Zhang and J. Wang, J. Org. Chem., 2014, 79, 8689 CrossRef CAS PubMed; (p) F. Hu, Y. Xia, Z. Liu, C. Ma, Y. Zhang and J. Wang, Org. Biomol. Chem., 2014, 12, 3590 RSC; (q) Z. Liu, L. Wang, H. Tan, S. Zhou, T. Fu, Y. Xia, Y. Zhang and J. Wang, Chem. Commun., 2014, 50, 5061 RSC; (r) F. Hu, Y. Xia, F. Ye, Z. Liu, C. Ma, Y. Zhang and J. Wang, Angew. Chem., Int. Ed., 2014, 53, 1364 CrossRef CAS PubMed; (s) M. Hossain, F. Ye, Y. Zhang and J. Wang, J. Org. Chem., 2013, 78, 1236 CrossRef CAS PubMed; (t) Z. Liu, Y. Zhang and J. Wang, Chin. J. Org. Chem., 2013, 33, 687 CrossRef CAS; (u) Q. Xiao, Y. Zhang and J. Wang, Acc. Chem. Res., 2013, 46, 236 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra12099c |
| This journal is © The Royal Society of Chemistry 2015 |