Chemical fractionation of a terrestrial humic acid upon sorption on alumina by high resolution mass spectrometry†
Abstract
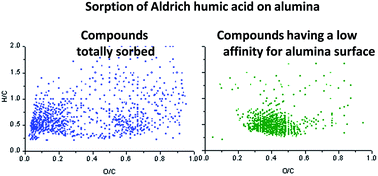
Understanding the fractionation of humic acids (HA) during their sorption at mineral-solution interfaces is one of the major issues of soil and environmental sciences. Molecular-scale investigations have been conducted on the fractionation of a terrestrial HA (more precisely, of the water-soluble fraction of Aldrich HA denoted WSAHA) – rich in highly condensed aromatic compounds – during its sorption at acidic pH on alumina particles, which were taken as surrogates of Al oxide hydrates existing in soils. High-resolution mass spectrometry combined with electrospray ionization and atmospheric pressure chemical ionization was used for analysing WSAHA solutions before and after their contact with alumina. The sorption process was found to lead to enrichment of the highly reactive, acidic, oxygen-functionalized aromatic and aliphatic molecules, and of highly condensed aromatic compounds depleted in hydrogen carrying only a few oxygenated groups on the alumina surface. In contrast, the poorly oxygenated aliphatic constituents and aromatic compounds of O/C values in the range 0.2 to 0.5 remained preferentially in the solution. By comparing results obtained for homologous compounds whose elemental composition differed only by the number of CO2 groups, evidence is found that both molecular acidity and degree of molecular hydrophobicity influence the degree of sorption (via ligand exchange on the surface and hydrophobic interactions, respectively) of WSAHA compounds of highly condensed aromatic type. Evidence at the molecular scale is provided that molecular acidity and hydrophobicity are the determining factors that control the size-fractionation of WSAHA during sorption on alumina.

 Please wait while we load your content...
Please wait while we load your content...