A new electrochemical sensor based on a nitrogen-doped graphene/CuCo2O4 nanocomposite for simultaneous determination of dopamine, melatonin and tryptophan
F. Tadayon*a and
Z. Sepehrib
aDepartment of Chemistry, Islamic Azad University, North Tehran Branch, Tehran, Iran. E-mail: f_tadayon@iau-tnb.ac.ir
bDepartment of Internal Medicine, Zabol University of Medical Sciences, Zabol, Iran
First published on 27th July 2015
Abstract
A new nanocomposite based on nitrogen-doped graphene nanosheets/CuCo2O4 nanoparticles was prepared and used as an electrode material for simultaneous determination of dopamine, melatonin and tryptophan. For this purpose, CuCo2O4 nanoparticles were supported on porous nitrogen-doped graphene nanosheets by a simple method. The nanocomposite was characterized by transmission electron microscopy and X-ray diffraction spectroscopy. Incorporation of the prepared nanocomposite in a carbon paste electrode (CPE) increased the oxidation peak currents and reduced the overpotential of dopamine, melatonin and tryptophan. The new developed sensor was then employed for the simultaneous determination of target analytes with linear ranges of 0.010–3.0 μM, using differential pulse voltammetry. Detection limits of 0.0033, 0.0049 and 0.0041 μM were achieved for dopamine, melatonin and tryptophan, respectively. The selectivity of the method was studied and the results showed that the fabricated sensor is free from interference of organic compounds especially uric acid and ascorbic acid. Finally, the proposed electrochemical sensor was employed to determine analytes in urine, serum and pharmaceutical samples.
Introduction
Over the past decades, to meet the growing demands for ultrasensitive detection, researchers have developed many techniques to enhance the response of electrochemical sensors by modifying them with different functional materials. With the continuous development of nanotechnology, great attention has been paid to different nanomaterials, such as carbon-based nanomaterials, metal nanoparticles (NPs), quantum dots, magnetic NPs and polymeric NPs.1–4 Because of their biological compatibility, high surface area, chemical stability, excellent catalytic activity and conductivity, the introduction of nanomaterials has really improved the analytical performance of the sensing strategies to obtain amplified detection signals and a stabilized sensing interface with good selectivity.1–5As a two-dimensional single-atom-thick carbon network, graphene (G) has been a research focus due to its unique structure and physiochemical properties.6 G has exhibited specific characteristics including extremely electric conductivity, large specific surface area, unusual electronic structure, and upstanding thermal conductivity.6–10 The production of G by the reduction of G oxide (GO) in chemical way produce hydroxyl (–OH) and carboxylate (–COOH) groups in the structure. These active functional groups enable the G structure to interact with metal NPs. These unique properties make G very useful for supporting metal NPs, and the obtained metal-NPs/G nanocomposites exhibit the synergistic effects and good sensitivity in their electrochemical detection behavior.11,12 The catalytic activity of the metal-NPs upon the electron transfer process paves the way for the production of metal-NPs/G composite based electrochemical sensors.12,13 However, G sheets tend to irreversible agglomeration and re-stacking because of their strong van der Waals interactions and π–π stacking,14 which causes some loss of performance for metal-NPs/G nanocomposites in the detection process. Furthermore, low reduction degree (especially for chemically reduced GO) and no-doping properties will also influence their efficiency.15,16 Thus, the prevention of aggregation and improvement of reduction degree and doping properties are greatly important for reduced graphene oxide (R-GO) in the preparation processes of metal-NPs/G nanocomposites. Nitrogen-doped graphene (N-G) can further improve the reactivity and electrocatalytic performance of G by forming of a delocalized conjugated system with the sp2-hybridized carbon frameworks ascribed to the lone electron pairs of nitrogen atoms. Moreover, N-G provides abundant binding sites for non-covalent functionalization as well as the enhanced biocompatibility and sensitivity in biosensing applications.14–17 Also, the N-doping and high reduction degree properties of N-G as supporter can enhance their own electrical conductivities and improve their own crystal structure, the obtained metal NPs/N-G composites should possess excellent properties for sensing.14–17
Among the G based nanocomposite, there are seldom reports about G-spinel cobaltites composite. Complex oxides (containing two or more types of cations) with spinel structure are of intense interests in material research because of their remarkable optical, electrical, magnetic, catalytic properties and widespread applications in science and engineering. Among these, spinel cobaltites (MCo2O4, M = Cu, Mn, Ni, Zn, Mg, etc.) have recently drawn considerable attention by virtue of their superior physicochemical properties and tremendous potential for many technological applications, ranging from catalysts and sensors to electrode materials and electrochromic devices.18–20 The combination of N-G with CuCo2O4 NPs can result in the synergetic effects of two components and exhibit the enhanced performance. The N-G/CuCo2O4 with rough interface can provide sufficient spaces for the some redox reactions with fast electron transfer rate.
Dopamine (Dp), melatonin (Me) and L-tryptophan (Tp) are important biomolecules that coexist in the extracellular fluid of the central nervous system and serum. These compounds play implicit roles in neurochemistry and biomedicine. Dp is one of the most prominent catecholamines, functioning as a neurotransmitter in the central nervous system. Me, an indoleamine synthesized from an essential amino acid (Tp), is a hormone synthesized by the pineal parenchymal cells from serotonin by N-acetylation and O-methylation and secreted by them into the blood and the cerebrospinal fluid. Dp deficiency and variations in Me levels have been linked to Parkinson's disease. Also, abnormal metabolism of neurotransmitters, particularly DA and ML, is frequently observed in individuals with phenylketonuria and is believed to be involved.21–23
Tp is an essential amino acid for humans and a precursor for serotonin, Me and niacin. A significant correlation has been reported between plasma Tp concentrations and depressive illness.24,25 Recently, effects of Tp and tyrosine supplementation were studied to optimize treatment in phenylketonuria with Dp and Me as biomarkers.26 Therefore, development of a sensitive and selective method for the determination of Dp, Me and Tp is desirable.
To the best of our knowledge, no study has been published so far reporting the simultaneous determination of Dp, Me and Tp by using any kind of modified electrodes. These species always coexist in biological fluids, and it is very important to develop sensitive and selective sensors for their simultaneous determination of in analytical applications and diagnostic researches. In this work, a simple electrochemical method based on the differential pulse voltammetry (DPV) for the direct determination of Dp, Me and Tp at a N-G/CuCo2O4/CPE was proposed.
Experimental
Reagents and apparatus
All chemicals and reagents used in this work were of analytical grade and used as received without further purification. Paraffin oil and graphite powder were obtained from Merck Company and used as received. Dp, Me and Tp were prepared from Sigma-Aldrich. Deionized distilled water (DDW) was used to prepare all the solutions. The commercial pharmaceuticals available from a local pharmacy were subjected to the analysis. Britton–Robinson (B–R) solutions were prepared in DDW and were tested as the supporting electrolytes.Transmission electron microscopic (TEM) images were taken using Zeiss EM902A (Germany). X-ray powder diffraction (XRD, 38066 Riva, d/G.Via M. Misone, 11/D (TN) Italy) was employed to analyze the chemical components of the composites. Electrochemical experiments were carried out at room temperature using a Behpajoh potentiostat/galvanostat system (model BHP-2065). The electrochemical cell was assembled with a conventional three electrode system: a saturated calomel electrode (SCE) as a reference electrode (Azar electrode) and a platinum disk as an auxiliary electrode. Different working electrodes including CPE and modified CPEs were used. The pH measurements were carried out using a Metrohm pH meter (model 713) with a combined pH glass electrode.
Preparation of nanocomposite
Results and discussion
Characterization of materials
The size, morphology and structure of the resulting materials were investigated by TEM. The morphology of GO, consisting of thin stacked flakes and having a well defined few-layer structure at the edge, can be clearly seen in Fig. 1a. Typical TEM micrograph of CuCo2O4 (Fig. 1b) shows the formation of uniform and monodispersed CuCo2O4 NPs with mean diameter and length about 15–25 nm. As shown in Fig. 1c, CuCo2O4 NPs are grown on a 3D flame like N-RGO and G sheets are completely covered with NPs.The phase structure of synthesized samples was determined by XRD. The XRD patterns of N-RGO, CuCo2O4 NPs and N-RGO/CuCo2O4 are shown in Fig. 1d. The synthesized GO displayed a typical characteristic (002) peak at 2θ = 10°.The crystal structure of CuCo2O4 is in a cubic structure and the fitted lattice parameter of 8.131(5) are in good agreement with the standard JCPDS card no. 78-2177 (a = 8.133 Å). For N-RGO/CuCo2O4 the diffraction peak arising from GO nearly disappeared at 2θ = 10°, suggestive of less agglomeration of the G sheets in the nanocomposite. All of the other diffraction peaks can be indexed to the structure of CuCo2O4 in nanocomposite.
Electrochemical impedance spectroscopy (EIS)
Fig. 2 exhibits the Nyquist plots of the CPE, G/CPE, Cu2Co2O4/CPE and N-RGO/CuCo2O4/CPE. The CPE (curve a) revealed a very large semicircle domain, implying a very high electron transfer resistance (Ret) of the redox probe. After CPE was modified with Cu2Co2O4 (curve b) or G (curve c), the Ret was a small semicircle domain, showing that the G/CPE and Cu2Co2O4/CPE promoted conductivity. When the electrode was conjugated with N-RGO/CuCo2O4/CPE (curve d), the Ret again decreased. This was attributed to that the coating of N-RGO surface with CuCo2O4 nanoparticles increased the ability of the redox probe to electron transfer. It can be seen that the electron transfer resistance decreased in the order of: bare CPE < Cu2Co2O4/CPE < G/CPE < N-RGO/CuCo2O4/CPE, which implying that CuCo2O4 and G were excellent electric conducting materials and accelerated the electron transfer.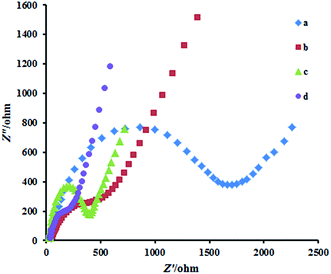 | ||
| Fig. 2 Nyquist plots for CPE (a) Cu2Co2O4/CPE (b) G/CPE (c) N-RGO/CuCo2O4 (d) in the presence of 1.0 mM [Fe(CN)6]4−/3− in B–R buffer solution (pH 7.0) with 0.1 M KCl. | ||
Electrochemical behavior of analytes
CVs of Dp, Me and Tp at CPE, G/CPE, CuCo2O4/CPE and N-RGO/CuCo2O4/CPE in buffer solutions (pH = 3.0) are shown in Fig. 3. At CPE, the peaks of Dp, Me and Tp are rather broad and weak, indicating a slow process of electron transfer (curve b in Fig. 3A–C). For G/CPE and CuCo2O4/CPE, peaks with defined shape and with the large peak currents were observed (curve c and d). The large oxidation peak current and shift in peak potential to less positive potentials for these electrodes as compared to the CPE indicates the electrocatalytic effect of modified surfaces. Moreover, at N-RGO/CuCo2O4/CPE, oxidation and reduction peaks of Dp are located at 457 mV and 151 mV showing a smaller peak-to-peak separation than that at CPE. The oxidation peak current is 5.5 times higher than that CPE suggesting the excellent electrocatalytic activity of N-RGO/CuCo2O4 toward Dp. The similar electrocatalytic activity for Me is also found, which occurs with an increased peak current (5.1 times) and positive shift compared with bare CPE, indicating good electron transfer promotion ability of N-RGO/CuCo2O4/CPE, also the oxidation peak current of Tp is 9.8 μA, which is about 5.8 times higher than that at CPE. Insets of Fig. 3 show CVs for N-RGO/CuCo2O4/CPE in absence of analytes.Fig. 4 shows the DPV responses at various electrodes in a ternary mixture solution of Dp, Me and Tp with 2.0 μM in B–R buffer solution (pH 3.0). At the CPE only a broad and overlapped oxidation peak is obtained and the potentials of Me and Tp are indistinguishable so that simultaneous determination of these biological molecules is impossible (Fig. 4b). However, at the N-RGO/CuCo2O4/CPE (Fig. 4e), three sharp and well defined oxidation peaks with larger peak separation potential and higher peak currents corresponding to the oxidation of Dp, Me and Tp were appeared. The three oxidation peaks for Dp, Me and Tp are well resolved at 440, 690 and 840 mV, respectively, with the peak separation potential is 250 mV (Dp–Me) and 150 mV (Me–Tp). All the voltammetric responses can demonstrate that the N-RGO/CuCo2O4/CPE possess excellent electrocatalytic activities towards the oxidation of Dp, Me and Tp, which can be attributed to its unique structural features and excellent electrochemical properties.29
Effect of pH and supporting electrolyte
A series of supporting electrolytes were tested (B–R, phosphate and acetate buffer solutions). Both the peak height and the peak shape were taken into consideration when choosing the supporting electrolyte. Of these, B–R buffer solution gave the best response.The effect of pH on the response of Dp, Me and Tp evaluated using DPV employing N-RGO/CuCo2O4/CPE. The effect of pH on peak potential and peak current of Dp, Me and Tp was investigated over the pH range of 2–9 employing B–R buffer solutions. As shown in Fig. 5b, for Dp, with increasing pH, the current increases and reaches a maximum value at pH = 5.0 and decreases with increasing in pH values. The peak currents of Me and Tp initially go up with increasing pH (=3), and then decreases with increasing pH (>3). In addition, Fig. 5c shows that the peak potential of Dp, Me and Tp is also pH dependent and the potentials shifted negatively when the pH of the solutions increases due to the participation of protons in the electrode reaction. The potentials of Dp, Me and Tp followed the linear regression equations with pH: Epa (V) = −0.0602 pH + 0.708 (R = 0.990), Epa (V) = −0.0264 pH + 0.748 (R = 0.984) and Epa (V) = −0.0520 pH + 1.130 (R = 0.992), respectively. For Dp and Tp the slopes is closed to the theoretical value of 59 mV per pH at 25 °C expected from the Nernst equation, indicates that the oxidation process is proton dependent and the electron transfer is accompanied by the transfer of an equal number of protons. Two electrons and two protons are involved for the oxidation of Dp and Tp. In the case of Me, the slope of 0.0264 V pH obtained on N-RGO/CuCo2O4/CPE was almost similar to that shown in previous electrochemical investigations and it is suggested that the number of electrons transferred in the oxidation of Me is double that of protons.30,31 The reaction mechanism for the oxidation of Dp, Me and Tp is as given in Scheme 1.
Effect of scan rate on the electrochemical oxidation of Dp, Me and Tp
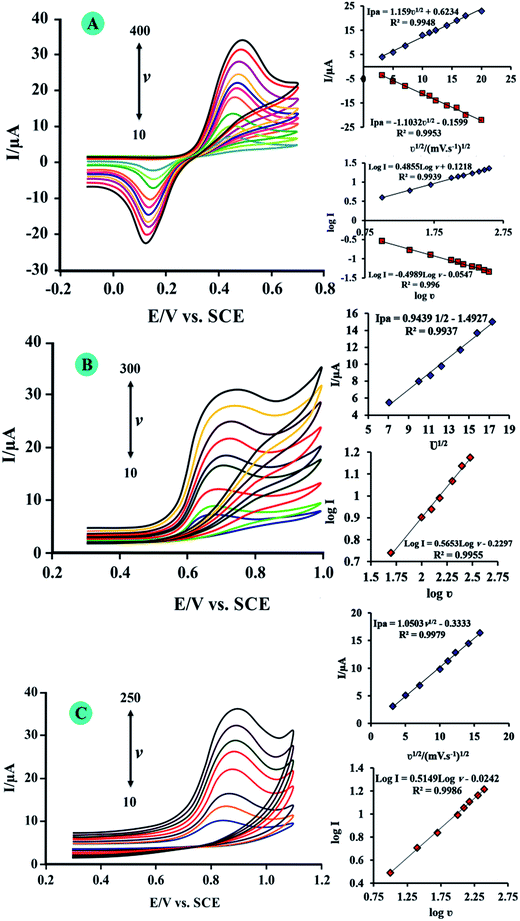
To investigate the reaction kinetics, the effect of scan rate on the peak currents and peak potentials of Dp, Me an Tp was studied (Fig. 6). Insets of Fig. 6 show that the peak currents changed in a linear relationship with the square root of scan rates in ranges of 10–400, 10–300, and 10–250 mV s−1 for Dp, Me and Tp, respectively, and by R-squared values of above 0.99 for all analytes. These plots indicate that the electrochemical reactions for these molecules are diffusion controlled. Also for more investigations, log peak currents versus log scan rates were plotted for analytes. For these plots when the slope is 0.5, the electrochemical reaction is a diffusion controlled process, and when equals to 1, the electrochemical reaction occurs via an adsorption-controlled process.32 From insets of Fig. 6 the plots of log anodic peak currents versus log scan rates have slopes of 0.485, 0.565 and 0.514 for Dp, Me and TP, respectively. These values show that the electrochemical reactions for these biomolecules are governed by diffusion control and the surface of N-RGO/CuCo2O4/CPE was not fouled by them.Individual and simultaneous voltammetric determination of Dp, Me and Tp
The above findings enabled us to develop a simple and suitable method for Dp, Me and Tp determination using N-RGO/CuCo2O4/CPE. As Fig. 7A–C shown, when detecting the individual, the concentration of one substance was changed with the others remained constant. Keeping the concentration of Me and Tp both at 2.0 μM, the peak current increased proportionally with adding concentrations of Dp from 0.010 to 3.0 μM. A linear regression equation between the oxidation peak current with the concentration of Dp was obtained Ip = 14.98C + 0.4382 (Ip in μA, C in μM) with R2 = 0.9997. The detection limit for Dp was calculated to be 0.0032 μM. Similarly, to keep the concentration of Dp and Tp both at 2.0 μM, the concentration of Me was changed within the range of 0.010 to 3.0 μM. The linear regression equation of Me was got: Ip = 10.00C + 0.507 with R2 = 0.9993. The detection limit for Me was calculated to be 0.0049 μM. In the same way, the concentration of Dp and Me remained were kept at 2.0 μM and the concentration range of Tp was from 0.01 to 3.0 μM, the linear regression equation of Tp was: Ip = 11.94C + 0.492 with R2 = 0.9907 and the detection limit for Tp was 0.0041 μM.The DPVs for different concentrations of Dp, Me and Tp were illustrated in Fig. 8. The resulting calibration plots are linear over the range from 0.01 to 3.0 μM for Dp, Me and Tp. The calibration curves and correlation coefficients are
| Dp:Ip (μA) = 14.71CDp (μM) + 0.521 R2 = 0.999 |
| Me:Ip (μA) = 9.95CMe (μM) + 0.509 R2 = 0.997 |
| Tp:Ip (μA) = 11.97CTp (μM) + 0.516 R2 = 0.998 |
The limits of detection were 0.0033 μM for Dp, 0.0049 μM for Me and 0.0041 μM for Tp based on 3sb/m, where sb is the standard deviation of the mean value for 5 independent voltammetric response of the blank solution.
Interferences, stability and reproducibility studies
Several compounds and ions from common co-existing substances were selected to evaluate the anti-interference ability of N-RGO/CuCo2O4/CPE. The effect of foreign compounds were tested by analyzing a standard solution of 1.5 μM Dp, 1.0 μM Me and 2.0 μM Tp. The tolerance limit is defined as the maximum concentration of influence substances which causes a ±5% relative error. It is found that species like glucose, lactose and sucrose, uric acid, ascorbic acid (till 100 fold excess), Na+, K+, Mg2+, Al3+, CO32−, NO3−, ClO4−, SCN−(till 1000 fold excess) did not interfere in the analysis of Dp, Me and Tp. The interference caused by same concentration of tyrosine was serious on tryptophan signal. Because tyrosine has similar electroactive group as tryptophan, its oxidation potential is close to tryptophan and thus affect the oxidation peak current of tryptophan. Hence determination of Dp, Me and Tp was not considerably affected by common interfering species, which shows that the method is more selective towards the drugs. The stability of the electrode was also tested. The peak current only decreased less than 5% after the electrode was stored at room temperature for 22 days. In addition, in order to evaluate the reproducibility of the modified electrode, a series of five N-RGO/CuCo2O4/CPEs were prepared for the detection of 2.0 μM of analytes. The RSD (n = 5) of peak currents for Dp, Me and Tp were calculated as 3.3%, 3.5% and 3.1%, respectively, which suggests that the reproducibility of the proposed electrode was good. The above results indicate that the as-obtained N-RGO/CuCo2O4/CPE presents well anti-interference ability, stability and reproducibility.Determination of Dp, Me and Tp in pharmaceutical, urine and serum samples
To confirm the practical usefulness of this proposed method for simultaneous determination of Dp, Me and Tp in pharmaceutical samples and biological fluids such as human urine, serum, Dp injection and Me tablet were selected as real samples for analysis using the standard addition technique. For sample pretreatments, both urine and serum samples were centrifuged at 5000 rpm for 3 min and the supernatants were collected, finally the prepared samples were diluted 50 times with B–R buffer solution (pH 3.0).The results are summarized clearly in Table 1, with the recoveries of the spiked samples ranged from 96.0% to 104.0%. It was evident that the N-RGO/CuCo2O4/CPE could be successfully applied for the simultaneous determination of Dp, Me and Tp in real samples. Moreover, the proposed method has good figures of merits in comparison with other reported methods in literatures. Some figures of merit related to previous reports and the present study are shown in Table 2.
| Samples | Analyte | Added (μM) | Found (μM) | Recovery (%) |
|---|---|---|---|---|
| Urine | Dp | 1.00 | 0.970 | 97.0 |
| 2.00 | 2.05 | 102 | ||
| Me | 1.00 | 1.01 | 101 | |
| 2.00 | 2.06 | 103 | ||
| Tp | 1.00 | 0.980 | 98.0 | |
| 2.00 | 2.04 | 102 | ||
| Human serum | Dp | 1.50 | 1.46 | 97.3 |
| 2.50 | 2.45 | 98.0 | ||
| Me | 1.50 | 1.45 | 96.6 | |
| 2.50 | 2.57 | 102 | ||
| Tp | 1.50 | 1.53 | 102 | |
| 2.50 | 2.48 | 99.2 | ||
| Dp injection | Dp | 0.000 | 0.810 | — |
| 0.500 | 1.29 | 97.5 | ||
| 1.20 | 2.03 | 102 | ||
| Me | 0.500 | 0.520 | 104 | |
| 1.20 | 1.24 | 103 | ||
| Tp | 0.500 | 0.480 | 96.0 | |
| 1.20 | 1.18 | 98.3 | ||
| Me tablet | Dp | 0.500 | 0.510 | 102 |
| 1.20 | 1.22 | 102 | ||
| Me | 0.000 | 0.540 | — | |
| 0.500 | 1.05 | 102 | ||
| 1.20 | 1.73 | 98.1 | ||
| Tp | 0.500 | 0.510 | 102 | |
| 1.20 | 1.19 | 99.1 |
| Electrode | Method | Linear range (μM) | Detection limit (μM) | Simultaneously with other analytes | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| ML | DA | TP | ML | DA | TP | ||||
| G–Fe3O4/CPE | SWV | 0.02–5.8 | 0.02–5.8 | — | 0.0084 | 0.0065 | — | — | 6 |
| ZnO nanorods modified carbon paste electrode | SWV | N.R. | 0.3–10.0 and 10.0 to 100.0 | — | N.R. | 0.056 | — | Methionine and caffeine | 33 |
| Boron-doped diamond electrode | CV | 345–688 | — | — | 10.3 | — | — | — | 34 |
| MWNT/GCE | CV | 0.08–10 | — | — | 0.02 | — | — | — | 35 |
| Boron-doped diamond electrode | SWV | 0.5–4 | — | — | 0.11 | — | — | — | 27 |
| MWCNTs–CHNPs/CILE | DPV | 0.01–50 | — | — | 0.004 | — | — | L-Dopa | 36 |
| MnHCFPEDOT/GCE | CV | 100–4600 | — | — | 100 | — | — | Catechin | 37 |
| TiO2–GR/4-ABAS/GCE | DPV | — | 1–400 | 1–400 | — | 0.3 | 0.3 | — | 38 |
| GNPs/PImox | — | 5.0–268.0 | 3.0–34.0 and 84.0–464 | — | 0.08 | 0.7 | Ascorbic acid and uric acid | 39 | |
| AgNPs/rGO | LSV | — | 10–800 | 10–800 | — | 5.4 | 7.5 | Ascorbic acid, uric acid | 40 |
| GS–PTCA | DPV | — | 0.4–370 | 0.4–140 | — | 0.13 | 0.06 | Ascorbic acid, uric acid | 41 |
| MWCNT/GO | Amperometry | — | — | 0.05–4.25 | — | — | 0.008 | — | 42 |
| NiCo2O4/Nano-ZSM-5/GCE | DPV | — | 0.6–900 | 0.9–1000 | — | 0.5 | 0.7 | Ascorbic acid and uric acid | 43 |
| MIP/G | SWV | 0.05–100 | — | — | 0.006 | — | — | — | 44 |
| N-RGO/CuCo2O4 | DPV | 0.01–3.0 | 0.01–3.0 | 0.01–3.0 | 0.0049 | 0.0033 | 0.0041 | — | This work |
Conclusions
In this work, a novel sensor based on N-RGO/CuCo2O4 nanocomposite as modifier in CPE, has fabricated that provides an extremely sensitive and selective method for the simultaneous determination of Dp, Me and Tp. Both CV and DPV studies reveal that the potential separations between them were large enough to allow their simultaneous detection. Moreover, appreciable reproducibility and lower detection limits were attained for the three biomolecules at N-RGO/CuCo2O4/CPE. Hence, it was employed for the analysis of Dp, Me and Tp in human urine, serum and pharmaceutical samples. Recovery values ranging from 97–104% were obtained for real sample analysis. The cost-effectiveness and easy preparative method are added advantages of this sensor. All these factors amalgamate to make this sensor a potential candidate for the detection of these species in routine analysis.Acknowledgements
The authors gratefully acknowledge the support of this work by the North Tehran Branch, Islamic Azad University.References
- C. Zhu, G. Yang, H. Li, D. Du and Y. Lin, Anal. Chem., 2014, 87, 230–249 CrossRef PubMed.
- A. Walcarius, S. D. Minteer, J. Wang, Y. Lin and A. Merkoçi, J. Mater. Chem. B, 2013, 1, 4878–4908 RSC.
- A. Chen and S. Chatterjee, Chem. Soc. Rev., 2013, 42, 5425–5438 RSC.
- H. Bagheri, A. Afkhami, Y. Panahi, H. Khoshsafar and A. Shirzadmehr, Mater. Sci. Eng., C, 2014, 37, 264–270 CrossRef CAS PubMed.
- H. Bagheri, R. P. Talemi and A. Afkhami, RSC Adv., 2015, 5, 58491–58498 RSC.
- A. T. Lawal, Talanta, 2015, 131, 424–443 CrossRef CAS PubMed.
- H. Bagheri, A. Afkhami, P. Hashemi and M. Ghanei, RSC Adv., 2015, 5, 21659–21669 RSC.
- H. Bagheri, A. Afkhami, H. Khoshsafar, M. Rezaei, S. J. Sabounchei and M. Sarlakifar, Anal. Chim. Acta, 2015, 870, 56–66 CrossRef CAS PubMed.
- A. Afkhami, H. Khoshsafar, H. Bagheri and T. Madrakian, Sens. Actuators, B, 2014, 203, 909–918 CrossRef CAS PubMed.
- A. Afkhami, H. Khoshsafar, H. Bagheri and T. Madrakian, Anal. Chim. Acta, 2014, 831, 50–59 CrossRef CAS PubMed.
- H. Bagheri, S. M. Arab, H. Khoshsafar and A. Afkhami, New J. Chem., 2015, 39, 3875–3881 RSC.
- E. Er, H. Çelikkan, N. Erk and M. L. Aksu, Electrochim. Acta, 2015, 157, 252–257 CrossRef CAS PubMed.
- S. Pruneanu, F. Pogacean, A. R. Biris, M. Coros, F. Watanabe, E. Dervishi and A. S. Biris, Electrochim. Acta, 2013, 89, 246–252 CrossRef CAS PubMed.
- L. Meng, Y. Xia, W. Liu, L. Zhang, P. Zou and Y. Zhang, Electrochim. Acta, 2015, 152, 330–337 CrossRef CAS PubMed.
- Y. Wang, Y. Shao, D. W. Matson, J. Li and Y. Lin, ACS Nano, 2010, 4, 1790–1798 CrossRef CAS PubMed.
- S. Park, J. An, J. R. Potts, A. Velamakanni, S. Murali and R. S. Ruoff, Carbon, 2011, 49, 3019–3023 CrossRef CAS PubMed.
- H. Xu, J. Xiao, B. Liu, S. Griveau and F. Bedioui, Biosens. Bioelectron., 2015, 66, 438–444 CrossRef CAS PubMed.
- E. Alizadeh-Gheshlaghi, B. Shaabani, A. Khodayari, Y. Azizian-Kalandaragh and R. Rahimi, Powder Technol., 2012, 217, 330–339 CrossRef CAS PubMed.
- M. Salavati-Niasari, N. Mir and F. Davar, J. Phys. Chem. Solids, 2009, 70, 847–852 CrossRef CAS PubMed.
- J. Zhu and Q. Gao, Microporous Mesoporous Mater., 2009, 124, 144–152 CrossRef CAS PubMed.
- D. Bonnefont-Rousselot and F. Collin, Toxicology, 2010, 278, 55–67 CrossRef CAS PubMed.
- T. Meng, Z.-H. Zheng, T.-T. Liu and L. Lin, Neurochem. Res., 2012, 37, 1050–1056 CrossRef CAS PubMed.
- S. Yano, K. Moseley and C. Azen, J. Pediatr., 2013, 162, 999–1003 CrossRef CAS PubMed.
- M. Kameya, H. Onaka and Y. Asano, Anal. Biochem., 2013, 438, 124–132 CrossRef CAS PubMed.
- A. Coppen, E. Eccleston and M. Peet, The Lancet, 1972, 300, 1415–1416 CrossRef.
- S. Yano, K. Moseley and C. Azen, J. Pediatr., 2014, 165, 184–189 CrossRef CAS PubMed.
- W. S. Hummers Jr and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339–1339 CrossRef.
- R. Ning, J. Tian, A. M. Asiri, A. H. Qusti, A. O. Al-Youbi and X. Sun, Langmuir, 2013, 29, 13146–13151 CrossRef CAS PubMed.
- C. Wei, Q. Huang, S. Hu, H. Zhang, W. Zhang, Z. Wang, M. Zhu, P. Dai and L. Huang, Electrochim. Acta, 2014, 149, 237–244 CrossRef CAS PubMed.
- A. Levent, Diamond Relat. Mater., 2012, 21, 114–119 CrossRef CAS PubMed.
- W. Xiao-Ping, Z. Lan, L. Wen-Rong, D. Jian-Ping, C. Hong-Qing and C. Guo-Nan, Electroanalysis, 2002, 14, 1654–1660 CrossRef PubMed.
- A. A. Rafati, A. Afraz, A. Hajian and P. Assari, Microchim. Acta, 2014, 181, 1999–2008 CrossRef CAS.
- E. Molaakbari, A. Mostafavi and H. Beitollahi, Sens. Actuators, B, 2015, 208, 195–203 CrossRef CAS PubMed.
- A. T. Ball and B. A. Patel, Electrochim. Acta, 2012, 83, 196–201 CrossRef CAS PubMed.
- W. Qu, F. Wang, S. Hu and D. Cui, Microchim. Acta, 2005, 150, 109–114 CrossRef CAS.
- A. Babaei, A. R. Taheri and I. K. Farahani, Sens. Actuators, B, 2013, 183, 265–272 CrossRef CAS PubMed.
- T.-H. Tsai, Y.-C. Huang and S.-M. Chen, Int. J. Electrochem. Sci., 2011, 6, 3238–3253 CAS.
- C.-X. Xu, K.-J. Huang, Y. Fan, Z.-W. Wu, J. Li and T. Gan, Mater. Sci. Eng., C, 2012, 32, 969–974 CrossRef CAS PubMed.
- C. Wang, R. Yuan, Y. Chai, S. Chen, F. Hu and M. Zhang, Anal. Chim. Acta, 2012, 741, 15–20 CrossRef CAS PubMed.
- B. Kaur, T. Pandiyan, B. Satpati and R. Srivastava, Colloids Surf., B, 2013, 111, 97–106 CrossRef CAS PubMed.
- W. Zhang, Y. Chai, R. Yuan, S. Chen, J. Han and D. Yuan, Anal. Chim. Acta, 2012, 756, 7–12 CrossRef CAS PubMed.
- J. F. Han, Q. Q. Wang, J. F. Zhai, L. Hana and S. J. Dong, Analyst, 2015, 140, 5295–5300 RSC.
- B. Kaur, B. Satpati and R. Srivastava, New J. Chem., 2015, 39, 1115–1124 RSC.
- P. Gupta and R. N. Goyal, RSC Adv., 2015, 5, 40444–40454 RSC.
| This journal is © The Royal Society of Chemistry 2015 |