Anode hydrodynamics in bioelectrochemical systems†
Abstract
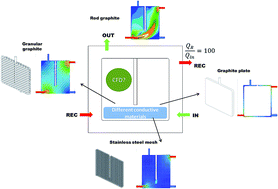
This study assesses the hydrodynamics in the anode compartment of a bioelectrochemical system (BES) when using different electrode materials (graphite rod, granular graphite, stainless steel mesh or graphite plate). For this purpose, computational fluid dynamics (CFDs) modelling was used. Granular graphite or stainless steel mesh allowed a better water flow distribution through the system favouring biomass attachment and consequently, removal efficiency and electricity production. This study provides the necessary mechanistic understanding on how these materials affect the hydrodynamics and substrate distribution behaviour within the bioanodes and electricity production.

 Please wait while we load your content...
Please wait while we load your content...