Highly robust tetrazolate based complexes for efficient and long-term stable dye sensitized solar cells†
Abstract
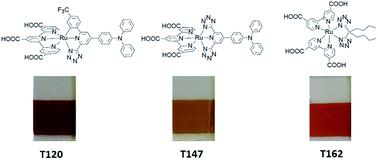
We report a new family of ruthenium poly-pyridyl complexes that bear tetrazolate based ligands (either bi-chelate as in T162 or tri-chelate as in T120 and T147), along with their spectroscopic, electrochemical, and theoretical characterization. Dye-sensitized solar cells (DSSCs) with these complexes show good conversion efficiencies that are highly dependent on the respective electrolyte composition especially in the case of T120 and T147, due to their low lying LUMOs when compared to N719 and T162. DSSCs based on these dyes showed superb stability under light soaking at 70 °C for 2000 h. The T120 and T147 based cells retained their initial efficiencies after the long term-stability test, while the T162 and N719 efficiencies decreased by 18% and 40%, respectively.

 Please wait while we load your content...
Please wait while we load your content...