β-Amylase production from packaging-industry wastewater using a novel strain Paenibacillus chitinolyticus CKS 1†
Katarina R. Mihajlovski*,
Neda R. Radovanović,
Miona G. Miljković,
Slavica Šiler-Marinković,
Mirjana D. Rajilić-Stojanović and
Suzana I. Dimitrijević-Branković
University of Belgrade, Faculty of Technology and Metallurgy, Department for Biochemical Engineering and Biotechnology, Karnegijeva 4, Belgrade, Serbia. E-mail: kmihajlovski@tmf.bg.ac.rs; Tel: +381-113303-788
First published on 12th October 2015
Abstract
Transport packaging factories generate a large amount of wastewater that contains residuals of starch glue. These residuals could be used as substrates for microorganism growth and enzyme production. In this study, β-amylase production using a new strain Paenibacillus chitinolyticus CKS1 was optimized using wastewater from a Serbian transport packaging factory. The optimization of β-amylase production was carried out using Response Surface Methodology (RSM). A Central Composite Design under the RSM with four interacting parameters (incubation time, inoculum concentration, casein hydrolysate concentration and yeast extract concentration) was employed to identify the optimal conditions for maximum β-amylase activity (334.20 U L−1) as 62 h of incubation with 2.40% inoculum, 2.02 g L−1 casein hydrolysate and 3.98 g L−1 yeast extract. High performance liquid chromatography showed that the P. chitinolyticus CKS1 strain hydrolyzed starch to form maltose as a major product. Due to the application of wastewater as an inexpensive material for enzyme and maltose production it may be considered that the economic and eco-friendly aspects of this method are very promising.
1. Introduction
Wastewater, as well as the waste itself, represents a significant source of environmental pollution. Serbian factories for the production of transport packaging, in terms of the protection of the environment, are not very representative, since wastewater with a high COD and BOD is currently released untreated. This wastewater contains significant concentrations of biodegradable organic matter, which consists of the remains of starch glue and cellulose fibers from paper residues that are used in the production of cardboard. The organic matter in wastewater from the packaging industry could, therefore, be used as a substrate for microbial growth, similar to that applied in the treatment of wastewater from the food industry.1,2 The application of such a biotechnological treatment to industrial wastewater facilitates a natural recycling process and typically results in the production of valuable products together with effluent purification.1–3The increasing concerns from environmental pollution have forced us to seek a cleaner industrial production method and to employ some specific enzymes which can significantly reduce pollution.4 In particular, nowadays, cellulases are used for the improved cellulose hydrolysis of lignocellulosic biomass,5,6 proteases have been used for the dehairing process,7 while laccases have a capability for dye decolouration8 and pollutant degradation.4,9
Similar to other enzymes, amylases could be used in wastewater treatment for diminishing starch residues. Amylases hydrolyze starch molecules to give diverse products including glucose, maltose and specific or mixed malto-oligosaccharides.10,11 Amylases can be divided into two categories, endoamylases and exoamylases.12 Endoamylases or α-amylase catalyze the hydrolysis of α-1,4-glycosidic linkages in a random manner in the interior of a starch macromolecule leading to the formation of oligosaccharides with varying lengths and α-limit dextrins, which constitute branched oligosaccharides. Exoamylases, either exclusively cleave α-1,4-glycosidic bonds such as β-amylase and produce maltose or cleave both α-1,4 and α-1,6-glycosidic bonds like amyloglucosidase or glucoamylase and α-glucosidase and produce glucose. To hydrolyze starch completely a combined action of various enzymes is required.12,13
β-Amylase is used for starch processing and its main application is for producing maltose syrup,14 a product that is widely applied in the food industry.15
Most industrial α-amylases are produced by various Bacillus spp. during growth in starch medium.16–18 The optimization of media components for amylase production using Bacillus spp. was studied thouroughly.10,19 Paenibacillus spp. are also amylase producers, and it has been shown that amylase production can be obtained with Paenibacillus spp. using commercial substrates20 and agro-industrial wastes.21 Furthermore, there are two reports showing the expression of the amylase gene from Paenibacillus spp.22,23
In the literature, there is no report of amylase production by P. chitinolyticus, but in our study we show, for the first time that this species can be used for amylase production from the wastewater of the transport packaging industry using the CKS1 strain. The aim of this study was to optimize the conditions of the utilization of the wastewater from the transport packaging industry for amylase production by P. chitinolyticus CKS1. The wastewater from the transport packaging industry was used as a model solution. Response surface methodology (RSM) using a Central Composite Design (CCD) was used for the optimization of fermentation parameters: incubation time, inoculum concentration, casein hydrolysate concentration and yeast extract concentration to obtain maximum β-amylase activity. Analysis of the end products of fermentation using high performance liquid chromatography (HPLC) showed that the treatment of wastewater using the CKS1 strain yields another valuable end product-maltose.
2. Experimental methods
2.1. Microorganisms
The CKS1 strain was isolated from a soil sample taken from a coniferous forest, from the foot of the Alps and identified as P. chitinolyticus based on the almost full-length 16S rRNA gene sequence (KP 715850).24 The reference strain was P. chitinolyticus DSM11030. Both microorganisms were cultured on ISP1 liquid medium which consisted of 5.0 g L−1 casein hydrolysate and 3.0 g L−1 yeast extract.The CKS1 strain and the reference strain were screened for amylase production on a starch agar plate containing 0.1 g L−1 starch and 0.1 g L−1 agar in ISP1 liquid medium. Five microlitres of tested bacterial strains, which had previously been grown in the liquid ISP1 medium, was spot plated on starch agar plates. After incubation for 24–48 h at 30 °C, the plates were flooded with Gram’s iodine (2 g of KI and 1 g of iodine in 300 mL of distilled water) for 3 to 5 minutes and observed for starch hydrolysis. The zone of clearance observed around the colonies indicated amylase activity.
2.2. Inoculum and medium preparation for amylase production
P. chitinolyticus CKS1 was grown in ISP liquid medium in a rotary shaker with a mixing speed of 150 rpm at 30 °C for 24 h.Wastewater, which was used for the amylase production medium, was obtained from a Serbian transport packaging factory. The composition and characteristics of the wastewater are provided in Table S1 (ESI† file 1).
BOD and COD analyses of wastewater were carried out using the Merck-Spectroquant BOD test 1.00687 and Merck-Spectroquant COD test 1.09773, respectively. Nitrates, nitrites and iron were analysed according to the Merck-Spectroquant Nitrate test 1.14773, Merck-Spectroquant Nitrite test 1.14776 and Merck-Spectroquant Iron test 1.00796, respectively. A gravimetric method was used to determine the total dissolved solids in the wastewater and an electrometric method was used to determine the pH value of the wastewater. Standard methods for the determination of metals in wastewater were described previously.25
The production medium contained the same ingredients ( 3 g L−1 yeast extract and 5 g L−1 casein hydrolysate) as the ISP medium, with the exception that wastewater was used instead of distilled water for medium preparation. After sterilization at 121 °C for 20 min, an overnight bacterial culture was inoculated into fresh medium in a rotary shaker with a mixing speed of 150 rpm at 30 °C. All fermentations were carried out in 300 mL Erlenmeyer flasks with 30 mL of production medium in an orbital shaker (150 rpm) at 30 °C. The culture medium was centrifuged at 6000 rpm for 15 min to remove the cells. The crude cell-free supernatant was analysed for β-amylase activity. The effect of culture passaging on β-amylase production was examined by transferring the inoculum of 3% culture every 24 h into fresh medium (passaging). Each passage was monitored for β-amylase activity for 4 days.
2.3. Enzyme test for amylase
The activity of amylase was measured using a modified Bernfeld method.262.4. Experimental design
Based on preliminary single factor experiments (data not shown) a CCD was chosen to examine the effect of four independent variables: incubation period (A), inoculum concentration (B), casein hydrolysate concentration (C) and yeast extract concentration (D) within the defined ranges that favored optimal feedback of the β-amylase production response. Each factor in this design was studied at five different levels (Table 1).| Factors | −1 | 0 | +1 | Axial (−α) | Axial (+α) |
|---|---|---|---|---|---|
| A: incubation period, h | 32 | 46 | 60 | 18 | 74 |
| B: inoculum, % | 3 | 4 | 5 | 2 | 6 |
| C: casein hydrolysate, g L−1 | 2 | 3.5 | 5 | 0.5 | 6.5 |
| D: yeast extract, g L−1 | 2 | 3.5 | 5 | 0.5 | 6.5 |
The data from the CCD were analysed using multiple regressions to fit to a second-order polynomial regression model containing the coefficient of linear, quadratic, and two factor interaction effects.
The model equation of the response (Y) of the four independent variables (A, B, C and D) is given in the following equation:
| Y = β0 + β1A + β2B + β3C + β12AB + β13AC + β23BC+ β11A2 + β22 B2 + β33C2 | (1) |
The RSM was applied using a statistical package, Design-Expert (Version 8, Stat-Ease, Inc., Minneapolis, United States).
2.5. HPLC analyses of starch hydrolyses
The starch hydrolysis product, obtained from the CCD with maximum β-amylase activity, was analyzed using high performance liquid chromatography (HPLC). 5.0 mL of enzyme solution (crude bacterial supernatant) was incubated at 50 °C with 5.0 mL of 1% (w/v) soluble starch solution made in 0.016 M sodium acetate buffer (pH 4.8). After different time intervals (15, 30, 60 and 120 min), samples were withdrawn and hydrolysis was stopped by boiling the samples for 5 minutes. The samples were then filtered through a 0.22 μm membrane filter.For quantitative analysis of the obtained samples, a Dionex Ultimate 3000 Thermo Scientific (Waltham, USA) HPLC system was used. A carbohydrate column (Hyper REZ XP Carbohydrate Ca2+, 300 mm × 7.7 mm, 8 μm) at 80 °C was employed. Water (HPLC grade, JT Baker (USA)) was used as the sole mobile phase with an elution rate of 0.6 mL min−1 during the analysis. Detection was performed using a RI detector (RefractoMax 520, ERC, Germany). All data acquisition and processing was done using Chromeleon Software. The separated hydrolysis products were identified by comparison with standard glucose, maltose, raffinose and dextrin and with the literature data of a used HPLC system for oligosaccharides. The soluble starch (Merck) solution was included as a control.
3. Results and discussion
3.1. Screening for amylolytic activity
Amylase production was indicated by the appearance of a halo around the bacterial colony, indicative of areas of hydrolysis. P. chitinolyticus CKS1 produced clear zones of 4.00 ± 0.29 mm in diameter. Reference strain P. chitinolyticus DSM 11030 showed modest amylolytic activity with a 0.50 ± 0.01 mm area of hydrolysis. The CKS1 strain was used in further investigations as it was identified as the potent amylolytic strain of the P. chitinolyticus species.3.2. pH influence on P. chitinolyticus CKS1 amylolytic activity
Testing the influence of pH on the amylolytic activity of the crude enzyme shows the presence of two peaks indicative of the presence of two amylolytic enzymes produced by P. chitinolyticus CKS1 (Fig. 1). The data indicate that one enzyme had an optimum activity at pH 4.8 and the other at pH 6.90. To determine the type of enzymes produced by the tested strains, the products of hydrolysis obtained with the crude enzyme at pH 4.80 and 6.90 were analysed using HPLC. The results indicate the predominant presence of maltose in hydrolysate obtained in solution with pH 4.80 with traces of other carbohydrates including glucose and longer oligosaccharides (Fig. S1†). Based on the literature data, and the hydrolysis products it was preliminarily concluded that when accessing the activity of the crude enzyme at pH 4.80, the enzymatic activity could predominantly be attributed to β amylase. | ||
| Fig. 1 Effect of pH on the amylolytic activity of the crude enzyme obtained on a starch substrate (a) and ISP medium with wastewater (b). | ||
3.3. Amylase production
Amylase production by P. chitinolyticus CKS1 was followed in media prepared with wastewater from the transport packaging industry supplemented with organic sources of nitrogen, yeast extract and casein hydrolysate. In order to achieve maximal hydrolysis, the hydrolysates obtained after 24 and 48 h of incubation were measured (Fig. 2). β-Amylases showed activity after 24 h of incubation of the CKS1 strain, that further increased with the increase of incubation time until 48 h and the value was 185.25 ± 1.89 U L−1. Compared to the activity of amylases produced by other bacteria, this is a dramatically lower value. However, one should keep in mind that the obtained amylase activity was also almost 10 fold lower than that obtained using starch as the inducer of amylase synthesis (Fig. 1). Amylases are inducible enzymes and for their production a source of carbon is required. In this study, wastewater was used as a substrate for microorganism growth and enzyme production. This wastewater contains only 0.1% suspended solids (Table S1†) which consist mainly of starch glue residues which serve as a source of carbon for microorganism growth and amylase production. Low values of amylase activity can be explained by the low concentration of starch in wastewater. In addition to the limited substrate amount, the wastewater could have contained various inhibitors of microbial growth (not measured) and/or a variety of toxic waste matter which may affect the growth of bacteria and enzyme production.Hernandez et al.1 studied the influence of the initial concentration of starch 10–40 g L−1 in brewery and meat processing wastewater on amylase production. This wastewater was supplemented with different starch concentrations and the highest amylase production of 70.29 EU mL−1 and 60.12 EU mL−1 was obtained in brewery and meat processing wastewater supplemented with 40 g L−1 starch, indicating the great influence of carbon (starch concentration) on enzyme production. However, since the goal of this study was to purify the wastewater in line with amylase production, no additional carbon sources were added into the wastewater.
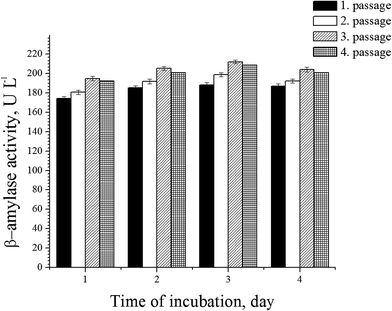
Subculturing (passaging) of a microorganism in a medium of essentially the same composition as that employed for the final culture has been an effective tool for enhancing a desired property.27 This indicates that a certain adaptation of the microorganism is required for the desired characteristic. In order to define if the adaptation of the microorganism in the medium for β-amylase production has an impact on amylase activity, the influence of passaging the culture microorganism was examined, and proved positive. β-Amylase activity increased with culture passaging and with incubation time (Fig. 3). The highest β-amylase activity was detected for the third passage and on the third day of incubation with a value of 212.11 ± 2.44 U L−1. A slight decrease in β-amylase activity was observed in the fourth passage. Therefore, the second passage was used as the inoculum for further investigation of β-amylase production as this design enabled us to perform other tests with the third passage of the bacterial culture.
3.4. Fitting the process variables
A total of 30 randomized experiments, including six replicates as the centre points were assigned to evaluate the pure error (Table 2).| Run | Independent variable | Response | |||
|---|---|---|---|---|---|
| A (h) | B (%) | C (g L−1) | D (g L−1) | Y (U L−1) | |
| a A: incubation period; B: inoculum concentration; C: casein hydrolysate concentration; D: yeast extract concentration; Y: β-amylase activity. | |||||
| 1 | 60 | 5 | 5 | 5 | 231.086 |
| 2 | 60 | 5 | 5 | 2 | 281.263 |
| 3 | 32 | 3 | 5 | 2 | 117.211 |
| 4 | 60 | 3 | 5 | 5 | 223.194 |
| 5 | 60 | 5 | 2 | 2 | 205.684 |
| 6 | 60 | 5 | 2 | 5 | 177.076 |
| 7 | 46 | 4 | 3.5 | 3.5 | 226.154 |
| 8 | 32 | 3 | 5 | 5 | 171.921 |
| 9 | 60 | 3 | 2 | 2 | 258.215 |
| 10 | 32 | 3 | 2 | 2 | 182.191 |
| 11 | 32 | 5 | 2 | 2 | 155.978 |
| 12 | 46 | 4 | 3.5 | 3.5 | 218.262 |
| 13 | 60 | 3 | 5 | 2 | 141.241 |
| 14 | 32 | 3 | 2 | 5 | 265.121 |
| 15 | 46 | 4 | 3.5 | 3.5 | 231.351 |
| 16 | 32 | 5 | 5 | 5 | 202.725 |
| 17 | 46 | 4 | 3.5 | 3.5 | 226.647 |
| 18 | 32 | 5 | 5 | 2 | 235.526 |
| 19 | 60 | 3 | 2 | 5 | 322.520 |
| 20 | 32 | 5 | 2 | 5 | 106.048 |
| 21 | 46 | 4 | 3.5 | 6.5 | 134.903 |
| 22 | 46 | 4 | 6.5 | 3.5 | 119.612 |
| 23 | 46 | 4 | 3.5 | 0.5 | 93.7172 |
| 24 | 46 | 2 | 3.5 | 3.5 | 186.380 |
| 25 | 74 | 4 | 3.5 | 3.5 | 270.132 |
| 26 | 46 | 6 | 3.5 | 3.5 | 148.846 |
| 27 | 46 | 4 | 3.5 | 3.5 | 187.242 |
| 28 | 46 | 4 | 3.5 | 3.5 | 175.843 |
| 29 | 18 | 4 | 3.5 | 3.5 | 168.458 |
| 30 | 46 | 4 | 0.5 | 3.5 | 146.988 |
For the four examined factors the CCD model efficiently designed a second order response fit for the surface. The quadratic model was found to be the most suitable model. The ANOVA test of the significance of the regression model for the one response was evaluated (Table 3).
| F-Value | p-Value prob > f | |
|---|---|---|
| a Significant coefficient (P < 0.05).b Non-significant coefficient. | ||
| Model | 97.48864 | <0.0001a |
| A | 276.3014 | <0.0001a |
| B | 19.5155 | 0.0006a |
| C | 11.42568 | 0.0045a |
| D | 31.44915 | <0.0001a |
| AB | 0.217224 | 0.6483b |
| AC | 12.34753 | 0.0034a |
| AD | 0.177629 | 0.6798b |
| BC | 520.7398 | <0.0001a |
| BD | 223.2311 | <0.0001a |
| CD | 0.253497 | 0.6225b |
| A2 | 47.34463 | <0.0001a |
| B2 | 4.833885 | 0.0452a |
| C2 | 67.67623 | <0.0001a |
| D2 | 133.6956 | <0.0001a |
| Lack of fit | 1.63 | 0.3376b |
| R-Squared | 0.9898 | |
| Adjusted R-squared | 0.9797 | |
| Predicted R-squared | 0.9435 | |
| C.V.% | 3.85 | |
| Adequate precision | 42.683 | |
The second order equation was used to predict the maximum β-amylase production:
| Y = 203.29 + 25.29A − 6.72B − 5.14C + 8.53D − 6.55AC + 42.52BC − 27.84BD + 9.79A2 − 3.13B2 − 11.71C2 − 16.45D2 | (2) |
A positive sign in the equation represents a synergistic effect of the variables, while a negative sign indicates an antagonistic effect of the variables.
The significant factors (p-value<0.05) that influenced the response were A, B, C, D, the quadratic coefficients of A, B, C and D as well as interaction AC, BC and BD.
The analysis of variance (ANOVA) for the experimental results (Table 3) shows a small probability value (P < 0.001) indicating that the individual terms in the model are significant to the effect. The non-significant F-value for the lack of fit (1.63) compared with the pure error indicates that the model was adequate for predicting β-amylase production. The fit of the model was checked by calculating the determination coefficient (R-squared, adjusted R-squared and predicted R-squared). The value of R-squared is close to 1 for the model, which is very high and indicates a good correlation between the observed and the predicted values and a good fit with a low dispersion (Fig. 4).28,29 Actual values were the measured response data for a particular run, and the predicted values were evaluated from the model. The adequate precision value 42.683 was greater than 4 which indicates that the signal was adequate. The coefficient of variance (CV) defines the reproducibility of the model and is the ratio of the standard error of estimate to the mean value of the observed response. If the CV of the model is not greater than 10%, the model can be considered reproducible. The value of the coefficient of variance, 3.85, suggested that the model was reliable and reproducible.29,30
 | ||
| Fig. 4 Plot depicting the correlation between the measured and the model predicted values of β-amylolytic activity. | ||
3.5. Effects of process variables
Regression analysis revealed that the influence of the casein hydrolysate concentration (C) and yeast extract concentration (D) on β-amylase production was statistically significant (p < 0.05) but their interaction, CD, was not statistically significant (Table 3). Similarly, this can be applied to the incubation time (A) and the inoculum concentration (B) and their interactions. Interactions AC, BC, and BD were statistically significant as well as the quadratic parameters A2, B2, C2 and D2. Eqn (2) shows that the time of incubation (A) and yeast extract concentration (D) have a linear positive influence on β-amylase production while inoculum concentration (B) and casein hydrolysate concentration (C) have a significant negative linear effect. Out of the four quadratic parameters only A2 (incubation time) had a positive influence on β-amylase production. The influence of different variables on β-amylase production was in the following order: incubation time (A) > yeast extract concentration (D) > inoculum concentration (B) > casein hydrolysate concentration (C).The incubation time of P. chitinolyticus CKS1, which showed the most prominent influence, varied from 18–74 h (Table 2) and the maximum β-amylase production was obtained after 60 h (Run 19, Table 2 and Fig. 5). The decrease in enzyme yield after the optimum incubation period (60 h) might be a consequence of the denaturation or decomposition of amylase, due to interaction with other components in the culture medium.17
 | ||
| Fig. 5 Surface plot of the interactive effects of incubation time and casein hydrolysate concentration and AC on β-amylolytic activity. | ||
In general, the optimal incubation period depends on the culture characteristics and growth rate.17 P. amylolyticus produced a maximum α-amylase activity (80 U g−1 min−1) after 72 h of solid state fermentation while growing on wheat bran.21 An incubation period of 60 h for solid state fermentation using a cassava fibrous residue by Streptomyces erumpens MTCC 7317 was also reported to yield a maximum amylase activity (3457.67 U per gds).31 For solid state fermentation of agro-industrial residues by Bacillus megaterium B69 a maximum amylase production (1034 U g−1) was achieved after 84 h of incubation.32 The shorter time of incubation of 42 h, with maximum amylase activity (965.9 U mL−1) was achieved when Bacillus amyloliquefaciens was incubated on a combination of wheat bran and groundnut oil cake (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the substrate in submerged fermentation.17 In contrast, the longest reported optimal incubation time for α-amylase production was 180 h by Streptomyces rimosus during growth on a sweet potato residue as the substrate in SSF.33
1) as the substrate in submerged fermentation.17 In contrast, the longest reported optimal incubation time for α-amylase production was 180 h by Streptomyces rimosus during growth on a sweet potato residue as the substrate in SSF.33
The contour plots are not perfectly elliptical which indicates that there may be less interaction occurring among the independent variables corresponding to the response surfaces.34
The literature data for amylase production from wastewater or waste materials by Paenibacillus spp. is very limited and results are difficult to compare with each other due to the different growing conditions of different microorganisms,17,31,32 different substrates or waste materials,1,33 and different procedures and units used for expressing the enzymatic activity.17,35,36 Nevertheless, it should be noted that other studies typically report higher enzymatic activity than in our study. While relatively low amylolytic activity might be to some extent a characteristic of the P. chitinolyticus species, that is depicted as non-amylolytic in Bergey’s manual,37 one should keep in mind that the substrate concentrations in the waste material treated in this study are much lower than in other wastes typically used for amylase production.
In addition to the incubation time, the concentration of yeast extract had a profound effect and stimulated β-amylase production. In our experiment yeast extract concentrations varied from 0.50 to 6.5 g L−1 and the maximum β-amylase activity of 322.52 U L−1 was obtained with 5 g L−1 yeast extract (Run 19, Table 2). Generally, yeast extract is the main nutritional supplement which serves as a rich source of amino acids, vitamins, nitrogen and carbon for bacterial growth and thus in enzyme production. The concentration of 5 g L−1 yeast extract was also reported to yield maximum β-amylase production by a Streptomyces sp.36 The maximum α-amylase activity from Aspergillu soryzae was achieved using 4.5 g L−1 yeast extract,38 while 20.0 g L−1 yeast extract was needed for maximum amylase production by Bacillus circulans GRS 313.19 It is interesting to note that a relatively low yeast extract (0–1.0 g L−1) results in the maximum amylase activity of a highly potent Bacillus sp. α-amylase producer.35
The effect of casein hydrolysate, as another source of nitrogen, was tested and showed a negative effect both as a linear factor and interaction with incubation time (AC) (Fig. 5). Only when increased along with inoculum size did casein hydrolysate concentration have a positive effect on β-amylase production (Fig. 6). Maximum amylase production was obtained using 2 g L−1 casein hydrolysate (Run 19, Table 2). Casein hydrolysate is an excellent source of free amino acids and short peptide fragments, which are required by microorganisms for growth. Also, it contains traces of minerals and ions that could enhance enzyme secretion.39 While amylase activity in some fungal strains could be increased by using more N-sources like urea, casein acid hydrolysate, soybean meal hydrolysate and (NH4)2SO4,39,40 P. chitinolyticus CKS1 preferred yeast extract in combination with a smaller proportion of casein hydrolysate as the nitrogen source.
 | ||
| Fig. 6 Surface plot of the interactive effects of inoculum concentration and casein hydrolysate concentration (BC) on β-amylolytic activity. | ||
Another factor that significantly affected β-amylase production was the amount of inoculum that had a negative influence on β-amylase production. This factor had an additional negative effect on β-amylase production, if the increase of inoculum size was accompanied with increased yeast concentration (Fig. 7).
 | ||
| Fig. 7 Surface plot of the interactive effects of inoculum concentration and yeast extract concentration (BD) on β-amylolytic activity. | ||
It has been fairly well established that extracellular amylase secretion in microorganisms is substantially influenced not only by medium components including a carbon source and nitrogen source, but also by culture conditions including pH, temperature, dissolved oxygen, and inoculum density. The importance of inoculum size with regard to the microbial fermentation processes is generally accepted.34 As it is shown in Fig. 7, β-amylase production increased with decreases in the inoculum size from 5 to 3%, until reaching a certain percentage of inoculum at which enzyme productivity achieved the maximum level. This demonstrates that inoculum density does not exert an unlimited effect on the fermentation process. There is an optimum value to be achieved, and this appears to be dependent on the microbial species and fermentation system being utilized.34 Inoculum density is particularly important in the growth of sporulating bacteria41 such as Paenibacillus spp. and consequently can influence the production yield of β-amylase. The optimization of inoculum density is quite important, as a high inoculum density can reduce enzyme production due to competition for available nutrients. In a similar manner, a low density can result in a reduction of enzyme secretion, owing to a drop in cell numbers.34
3.6. Validation of the model
Model validation was also performed. For the optimization of β-amylase production the desirability function approach was employed. The desirability function in an ideal case should be equal to 1 but in practical situations should be close to 1. Design Expert provides five options – none, maximum, minimum, target and within range – for choosing the desired goal for each variable and response.29 The desired goal for β-amylase production was set to maximum.It was used for two points selected from the numerical optimization results (Table 4). The obtained value of the predicted and validated response shows that the estimated function may represent the experimental model and desired conditions indicating that the model was reliable.
| Sample | Incubation time, h | Inoculum, % | Casein hydrolysate, g L−1 | Yeast extract, g L−1 | β-Amylase activity, U L−1 | |
|---|---|---|---|---|---|---|
| Predicted | Validated | |||||
| 1 | 62.00 | 2.40 | 2.02 | 3.98 | 333.145 | 334.201 |
| 2 | 18.00 | 2.14 | 3.05 | 6.50 | 262.89 | 260.674 |
3.7. Hydrolysis products of β-amylase
P. chitinolyticus CKS1 β-amylase hydrolyzed starch to form maltose as a major product (ESI† file 2). This product was readily apparent even during the early stages of the reaction (15 min) and increased in concentration along with the time course of the reaction. Maltooligosaccharides (i.e., limit-dextrins), maltotriose, rafinose with minor amounts of glucose were also produced. Hyun et al.42 reported that the main product of starch hydrolysis by β-amylases of C. thermosulfurogenes was maltose. The appearance of maltose as the major hydrolysis product and the relatively small amount of glucose with Clostridium thermosulfurogenes SV2 imply that the amylase produced by this microorganism is of the β type.43 Amylase from Halobacillus sp. LY9 hydrolyzed soluble starch to form maltose as the main product with trace amounts of longer oligosaccharides.44 According to Hensley et al.,45 linear amylose chains (soluble starch) with odd numbers of glucose units are responsible for the small amounts of glucose and maltotriose formed when amylose is digested with β-amylase. Hence, the amylase from P. chitinolyticus CKS1 may preferentially cleave at the α-1,4-linkage from the non-reducing ends of the starch molecule, releasing maltose which indicates β-amylase activity. Given that all experiments were performed with crude, not purified enzyme, the traces of other carbohydrates in the HPLC profile could be explained by residual activity of α-amylase under pH conditions not favorable by this enzyme.4. Conclusions
In this study, a cleaner and environmentally friendly enzyme production using wastewater was demonstrated. The results show that wastewater from a transport packaging factory could be used as a substrate for microorganism growth and amylase production. This is the first application of a P. chitinolyticus strain for the production of amylases, which makes the reported results fundamental. The novel strain P. chitinolyticus CKS1 could produce α and β-amylase while growing on wastewater supplemented with yeast extract and casein hydrolysate. Considering that the major product of the β-amylase hydrolysis of starch is maltose, β-amylase production was studied in more detail. Conditions for β-amylase production were optimized using a CCD in RSM. This approach indicated that β-amylase activity was mostly affected by incubation time followed by yeast extract concentration, and the negative effects of inoculum size and casein hydrolysate concentration. The optimized conditions for obtaining the maximal β-amylase activity of 334.20 U L−1 were determined to be 62 h of incubation, 2.40% of inoculum, 2.02 g L−1 casein hydrolysate and 3.98 g L−1 yeast extract. This study shows that the use of wastewater for the production of β-amylase is a procedure that when applied would have a positive economic and environmental effect as it generates cleaner water, β-amylase and maltose as the major products of starch hydrolysis.Acknowledgements
The financial support for this investigation given by Ministry of Science and Education of the Republic of Serbia under project TR 31035 is gratefully acknowledged. The authors would like to thank the MSc student Milica Carević for performing HPLC analysis.References
- M. S. Hernández, M. R. Rodriguez, N. P. Guerra and R. P. Rosés, J. Food Eng., 2006, 73, 93–100 CrossRef PubMed.
- B. Jin, X. Yan, Q. Yu and J. H. van Leeuwen, Adv. Environ. Res., 2002, 6, 179–189 CrossRef CAS.
- F. F. C. Barros, A. N. Ponezi and G. M. Pastore, J. Ind. Microbiol. Biotechnol., 2008, 35, 1071–1078 CrossRef CAS PubMed.
- J. Liu, Y. Cai, X. Liao, Q. Huang, Z. Hao, M. Hu, D. Zhang and Z. Li, J. Cleaner Prod., 2013, 39, 154–160 CrossRef CAS PubMed.
- A. Culbertson, M. Jin, L. da Costa Sousa, B. E. Dale and V. Balan, RSC Adv., 2013, 3, 25960–25969 RSC.
- M. Adsul, B. Sharma, R. R. Singhania, J. K. Saini, A. Sharma, A. Mathur, R. Gupta and D. K. Tuli, RSC Adv., 2014, 4, 44726–44732 RSC.
- J. Abraham, T. Gea and A. Sánchez, J. Cleaner Prod., 2014, 74, 191–198 CrossRef CAS PubMed.
- L. Gioia, C. Manta, K. Ovsejevi, J. Burgueño, P. Menéndez and S. Rodriguez-Couto, RSC Adv., 2014, 4, 34096–34103 RSC.
- F. Spina, C. Cordero, T. Schilirò, B. Sgorbini, C. Pignata, G. Gilli, C. Bicchi and G. C. Varese, J. Cleaner Prod., 2015, 100, 185–194 CrossRef CAS PubMed.
- J. K. Roy and A. K. Mukherjee, Biochem. Eng. J., 2013, 77, 220–230 CrossRef CAS PubMed.
- A. Pandey, P. Nigam, C. Soccol, V. Soccol, D. Singh and R. Mohan, Biotechnol. Appl. Biochem., 2000, 31, 135–152 CrossRef CAS.
- R. Gupta, P. Gigras, H. Mohapatra, V. K. Goswami and B. Chauhan, Process Biochem., 2003, 38, 1599–1616 CrossRef CAS.
- M. J. van der Maarel, B. van der Veen, J. Uitdehaag, H. Leemhuis and L. Dijkhuizen, J. Biotechnol., 2002, 94, 137–155 CrossRef CAS.
- S. Teotia, S. Khare and M. Gupta, Enzyme Microb. Technol., 2001, 28, 792–795 CrossRef CAS.
- O. Gaouar, N. Zakhia, C. Aymard and G. Rios, Ind. Crops Prod., 1998, 7, 159–167 CrossRef CAS.
- H. Ashraf, J. Iqbal and M. Qadeer, Bioresour. Technol., 2003, 87, 57–61 CrossRef.
- D. Gangadharan, S. Sivaramakrishnan, K. M. Nampoothiri, R. K. Sukumaran and A. Pandey, Bioresour. Technol., 2008, 99, 4597–4602 CrossRef CAS PubMed.
- G. Rajagopalan and C. Krishnan, Bioresour. Technol., 2008, 99, 3044–3050 CrossRef CAS PubMed.
- G. Dey, A. Mitra, R. Banerjee and B. Maiti, Biochem. Eng. J., 2001, 7, 227–231 CrossRef CAS.
- K. Tsusaki, H. Watanabe, T. Yamamoto, T. Nishimoto, H. Chaen and S. Fukuda, Biosci., Biotechnol., Biochem., 2012, 76, 721–731 CrossRef CAS PubMed.
- U. Hameed, Z. Mahmood and M. Javed, Pak. J. Bot., 2012, 44, 341–346 Search PubMed.
- T.-H. Jeong, H.-O. Kim, J.-N. Park, H.-J. Lee, D.-J. Shin, H. B. Lee, S.-B. Chun and S. Bai, J. Microbiol. Biotechnol., 2001, 11, 65–71 CAS.
- T. Rajesh, Y. H. Kim, Y.-K. Choi, J. M. Jeon, H. J. Kim, S.-H. Park, H.-Y. Park, K.-Y. Choi, H. Kim and H. J. Kim, Appl. Biochem. Biotechnol., 2013, 170, 359–369 CrossRef CAS PubMed.
- K. R. Mihajlovski, M. B. Carević, M. L. Dević, S. Šiler-Marinković, M. D. Rajilić-Stojanović and S. Dimitrijević-Branković, Int. Biodeterior. Biodegrad., 2015, 104, 426–434 CrossRef CAS PubMed.
- M. Rand, A. E. Greenberg and M. J. Taras, Standard methods for the examination of water and wastewater, Prepared and published jointly by American Public Health Association, American Water Works Association, and Water Pollution Control Federation, 1976 Search PubMed.
- P. Bernfeld, Methods Enzymol., 1955, 1, 149–158 CAS.
- A. Boidin, and J. Effront, Process for treating amylaceous substances, US Pat., 1227374 A, 1917 Search PubMed.
- M. Ranic, M. Nikolic, M. Pavlovic, A. Buntic, S. Siler-Marinkovic and S. Dimitrijevic-Brankovic, J. Cleaner Prod., 2014, 80, 69–79 CrossRef CAS PubMed.
- A. T. Nair and M. M. Ahammed, J. Cleaner Prod., 2013, 96, 272–281 CrossRef PubMed.
- K. Adinarayana and P. Ellaiah, J. Pharm. Pharm. Sci., 2002, 5, 272–278 CAS.
- K. Shaktimay, T. K. Datta and R. C. Ray, Braz. Arch. Biol. Technol., 2010, 53, 301–309 Search PubMed.
- R. Saxena and R. Singh, Enzyme Res., 2014, 2014, 1–12 CrossRef PubMed.
- S.-S. Yang and J.-Y. Wang, Bot. Bull. Acad. Sin., 1999, 40, 259–265 CAS.
- L. Reddy, Y.-J. Wee, J.-S. Yun and H.-W. Ryu, Bioresour. Technol., 2008, 99, 2242–2249 CrossRef CAS PubMed.
- M. S. Tanyildizi, D. Özer and M. Elibol, Process Biochem., 2005, 40, 2291–2296 CrossRef CAS PubMed.
- M. Cotârleţ, Microbiology, 2013, 82, 147–154 Search PubMed.
- P. de Vos, G. Garrity, D. Jones, N. Krieg, W. Ludwig, F. Rainey, K.-H. Schleifer and W. Whitman, in Bergey’s Manual of Systematic Bacteriology, The Firmicutes, ed. W. B. Whitman, Springer, 2nd edn, vol. 3, 2009 Search PubMed.
- P. Gigras, V. Sahai and R. Gupta, Curr. Microbiol., 2002, 45, 203–208 CrossRef CAS PubMed.
- R. Kammoun, B. Naili and S. Bejar, Bioresour. Technol., 2008, 99, 5602–5609 CrossRef CAS PubMed.
- N. Nwe and W. F. Stevens, Process Biochem., 2004, 39, 1639–1642 CrossRef CAS.
- I. Mnif, S. Ellouze-Chaabouni and D. Ghribi, Probiotics Antimicrob. Proteins, 2013, 5, 92–98 CrossRef CAS.
- J. Zeikus, and H. Hyun, Thermostable beta amylase, US Pat., 4647538 A, 1987 Search PubMed.
- P. R. M. Reddy, M. Swamy and G. Seenayya, World J. Microbiol. Biotechnol., 1997, 14, 89–94 CrossRef.
- X. Li and H.-Y. Yu, J. Ind. Microbiol. Biotechnol., 2011, 38, 1837–1843 CrossRef CAS PubMed.
- D. E. Hensley, K. L. Smiley, J. A. Boundy and A. A. Lagoda, Appl. Environ. Microbiol., 1980, 39, 678 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra11964b |
| This journal is © The Royal Society of Chemistry 2015 |